38 lewis diagram for so2
Answer (1 of 2): Lewis Structure for SO2 (Sulfur Dioxide)||Lewis Dot Structure of SO2 (Sulfur Dioxide) Hello,today I am going to draw the lewis structure for SO2 in just five steps. Step-1: To draw the lewis structure for SO2, we have to find out the valence electrons of sulfur and oxygen firs... Here are the steps I follow when drawing a Lewis structure. > 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom ("S"). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-S-O". 3. Draw a trial structure by putting electron pairs around every atom until each gets an octet. In this editor, I will ...
Sulfur dioxide is a colorless gas with a pungent odor. It is a liquid when under pressure, and it dissolves in water very easily.Sulfur dioxide in the air comes mainly from activities such as the burning of coal and oil at power plants or from copper smelting. In nature, sulfur dioxide can be released to the air from volcanic eruptions.

Lewis diagram for so2
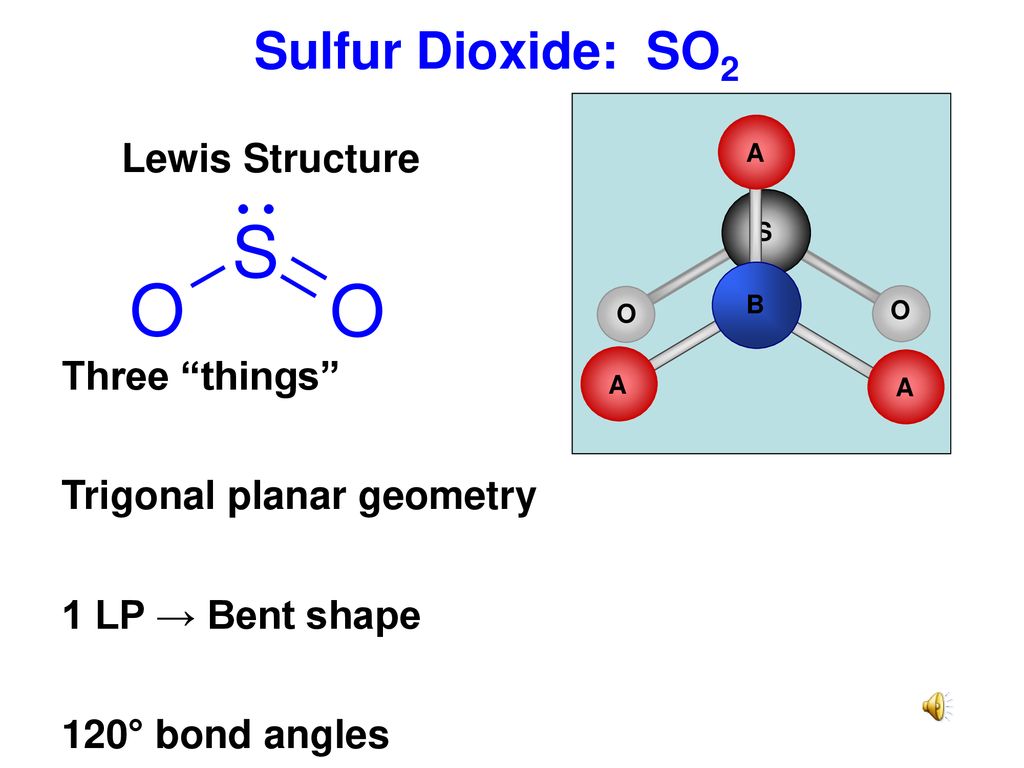
ClO2- Lewis Structure, Geometry, Hybridization, and Polarity. ClO2 is the molecular formula of Chlorine dioxide that is commonly used to treat potable water. It is far better than chlorine because it has higher solubility in water and does not hydrolyze unlike chlorine, and resides as dissolved gas. In ionic form chlorine dioxide is known as ... The Lewis dot structure of SO2 or sulfur dioxide has a central atom of sulfur that violates the octet rule. It causes a repulsion of electron pairs to form the 120-degree angleBy analyzing the Lewis structure of SO2 we can see that the SO2 is asymmetrical because it contains a region with different sharing. SO2 Lewis structure would comprise of two atoms of oxygen (O) and one sulfur atom. The number of valence electrons in both S and O atoms is six.Molecular Geometry of SO2: Trigonal planarNo of Valence Electrons in the molecule: 18Hybridization of SO2: sp2 hybridizationName of molecule: Sulfur dioxide
Lewis diagram for so2. A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or someth... Website-http://www.kentchemistry.com/links/bonding/LewisDotTutorials/SO2.htm I quickly take you through how to draw the Lewis Structure of SO2 (Sulfur Dioxid... The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur. SO2 Lewis Structure Before directly jumping into the lewis structure of SO2, let's have a quick discussion regarding the importance of lewis structure and the steps to draw it. Lewis structure is the distribution of the electrons around the atoms of a compound.
SeO2 Lewis Structure, Geometry, Hybridization, and Polarity. The chemical formula of selenium dioxide is SeO2. It is a unidimensional polymer chain having alternating selenium and oxygen atoms. This chemical compound is of great importance because of its corrosive nature for metals only when in contact with water. Silicon Dioxide (SiO2) Lewis Structure. The Lewis structure of SiO2 is identical to the Lewis structure of CO2. The only difference is that instead of carbon, silicon is used. One silicon atom is at the middle, with two oxygen atoms bound to it in a double bond. There are no lone pairs on the central atom of the SiO2 Lewis dot structure, SO2 Lewis Structure. Step 1 - Figuring out the total number of valence electrons in the molecule is the first and most remarkable step. While doing so, do take care of the +, - signs. A '+' sign implies losing electrons and '-' means gaining.; Step 2 - Next thing is determining the central atom. The atom with the highest number of binding locations is the central atom. The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms. It is known as a formula written as SO2. Here we will provide an explanation of SO2 molecular geometry, SO2 electron geometry, SO2 bond angle, and SO2 Lewis structure. The Lewis dot structure of SO2, or sulfur dioxide, has a ...
SO2 Lewis structure. To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur. To design the best Lewis structure, you also need to calculate the formal charge of every atom too. You know that both the Sulphur and Oxygen has six valence electrons each. Click to see full answer. Sulfur dioxide (SO 2) Lewis Structure, Hybridization. Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. We will construct the lewis structure of SO 2 molecule by following VSEPR theory rules and considering stability of intermediate structures. After obtaining the lewis structure of SO 2, we can determine the hybridization of atoms. Lewis Structure of SO2 [duplicate] Ask Question Asked 3 years, 11 months ago. Active 3 years, 11 months ago. Viewed 51k times 3 $\begingroup$ This question already has answers here: Hybridization of sulfur in sulfur dioxide (2 answers) Closed 3 years ago. What is the structure of ... Lewis structure of SO 3 2-(sulfite) ion Resonance structures of SO 3 2-ion. Change the location of double bond and lone pairs of molecule to draw resonance structures of SO 3 2-ion. Three stable resonance structures can be drawn of SO 3 2-ion.. Questions
How to draw the Lewis Structure of SO2 - with explanationCheck me out: http://www.chemistnate.com
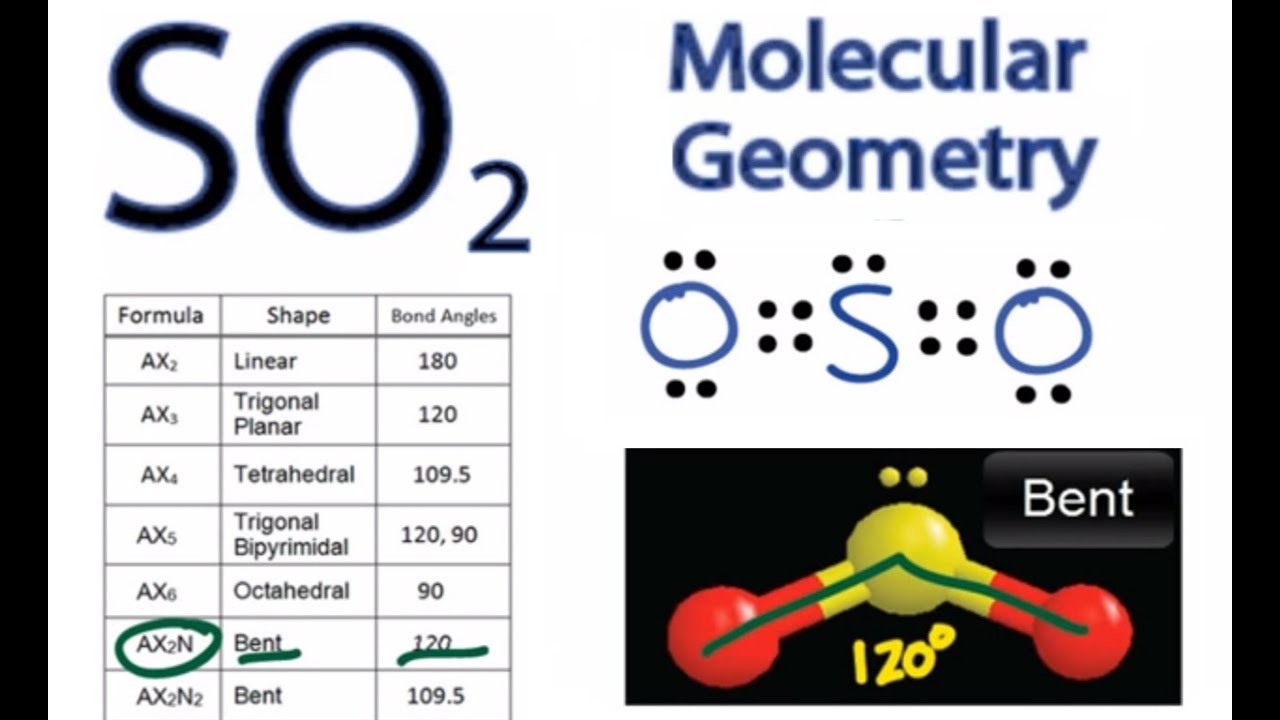
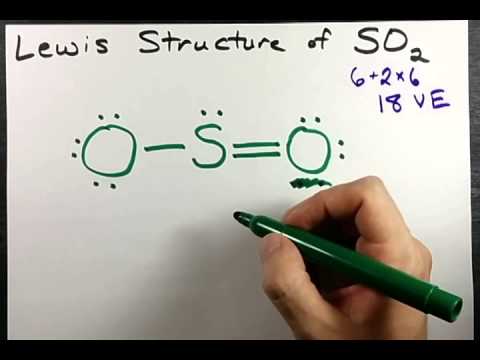
Let's do the SO2 Lewis structure. On the periodic table: Sulfur, 6 valence electrons. Oxygen has 6. We have two Oxygens, though, for a total of 18 valence electrons. We'll put the Sulfur in the middle; it's the least electronegative. Oxygens on the outside, and then we'll use our valence electrons. Two between atoms to form chemical bonds, and ...
By analyzing the Lewis structure of SO2, we can see that the SO2 is asymmetrical because it contains a region with different sharing. The molecular geometry of SO2 has a bent shape which means the top has less electronegativity, and the bottom placed atoms of Oxygen have more of it. So, the conclusion is, SO2 is a Polar molecule.

So2 Molecular Geometry Shape And Bond Angles Sulfur Dioxide In 2021 Molecular Geometry Molecular Molecules
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
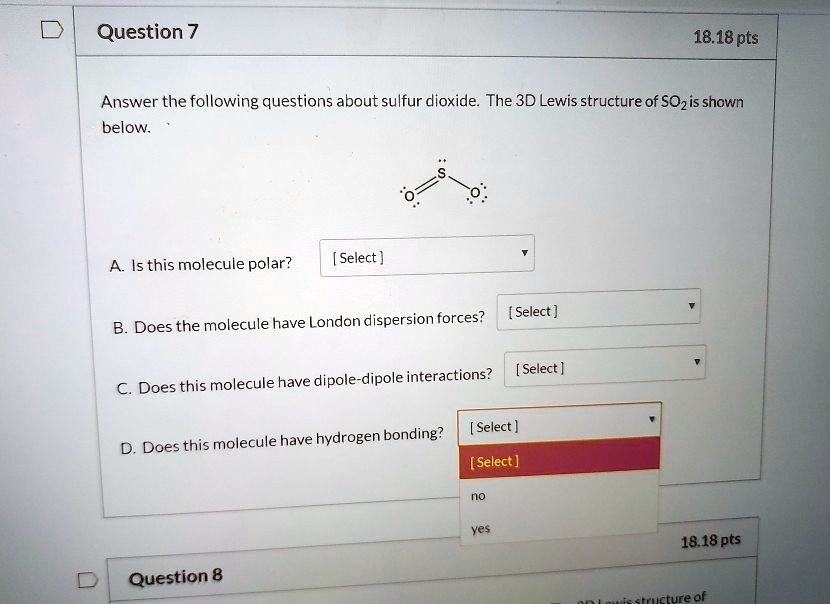
Solved Question 7 18 18 Pts Answer The Following Questions About Sulfur Dioxide The 3d Lewis Structure Of Sozis Shown Below Is This Molecule Polar Select Select Does The Molecule Have London
Answer (1 of 3): Do you mean the shape of a sulfur dioxide molecule? Please re-write the question. If that is the question it is bent linear
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2
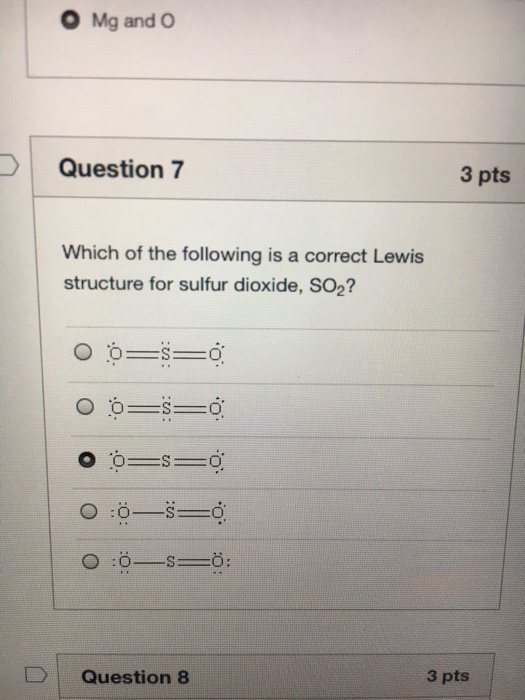
Chemistry. Chemistry questions and answers. Question 13 of 30 Draw the Lewis structure for SO2 (by following the octet rule on all atoms) and then determine the number of nonbonding electron pairs on the central atom. A) O B) 1 + C) 2 D) 4 E) 6 Click to draw a new structure.
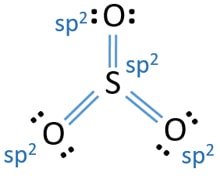
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2.
Carbon dioxide (CO 2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. Steps of drawing the lewis structure of CO 2 are explained in detail in this tutorial.
1 answerThe diagram of Lewis structure of SO2 S O 2 is given below. The central atom is the Sulfur (S) atom. Sulfur has 6 valence electrons and 4 electrons are ...
Lewis Structure. Popularly known as electron dot structure or Lewis dot structure is a representation of the bonds which are formed in between the elements of a molecule with the help of a diagram. You must also read out the article written on the lewis structure, geometry of SO2.
A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion).For the SO4 2- structure use the periodic table to find the total numb...
Aug 17, 2016 — To draw the lewis structure for SO2, we have to find out the valence electrons of sulfur and oxygen first.We express valence electrons as dots in lewis dot ...2 answers · 12 votes: * Decide which is the central atom in the structure. That will normally be the least electronegative ...Why is the Lewis structure of SO2 not similar to 03 ...2 answersNov 24, 2018Why does Sulphur in SO2 have 10 electron in the ...2 answersJan 19, 2017How is the molecular geometry for SO2 determined ...10 answersAug 4, 2016More results from www.quora.com
This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. It discusses the molecular geometry, bond angle,...
SO2 Lewis structure would comprise of two atoms of oxygen (O) and one sulfur atom. The number of valence electrons in both S and O atoms is six.Molecular Geometry of SO2: Trigonal planarNo of Valence Electrons in the molecule: 18Hybridization of SO2: sp2 hybridizationName of molecule: Sulfur dioxide
The Lewis dot structure of SO2 or sulfur dioxide has a central atom of sulfur that violates the octet rule. It causes a repulsion of electron pairs to form the 120-degree angleBy analyzing the Lewis structure of SO2 we can see that the SO2 is asymmetrical because it contains a region with different sharing.
ClO2- Lewis Structure, Geometry, Hybridization, and Polarity. ClO2 is the molecular formula of Chlorine dioxide that is commonly used to treat potable water. It is far better than chlorine because it has higher solubility in water and does not hydrolyze unlike chlorine, and resides as dissolved gas. In ionic form chlorine dioxide is known as ...






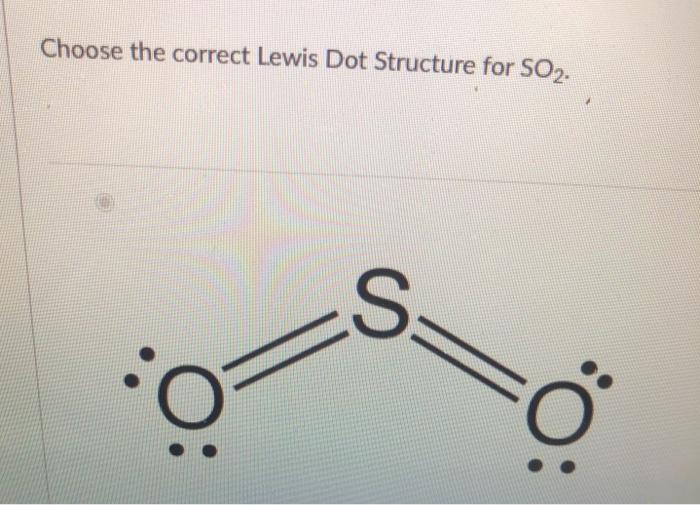





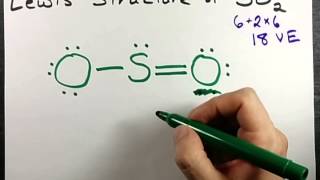







Comments
Post a Comment