39 potassium electron dot diagram
A step-by-step explanation of how to draw the Lewis dot structure for K (Potassium). I show you where Potassium is on the periodic table and how to determine... Electron Dot Diagram for Potassium. diagram electron dot diagram for potassium template information title electron dot diagram for potassium categories diagram ♦ publised friday january 27th 2017 01 49 18 am high school chemistry lewis electron dot diagrams since the lewis electron dot diagrams are based on the number of valence electrons it would hold true that the elements in the same ...
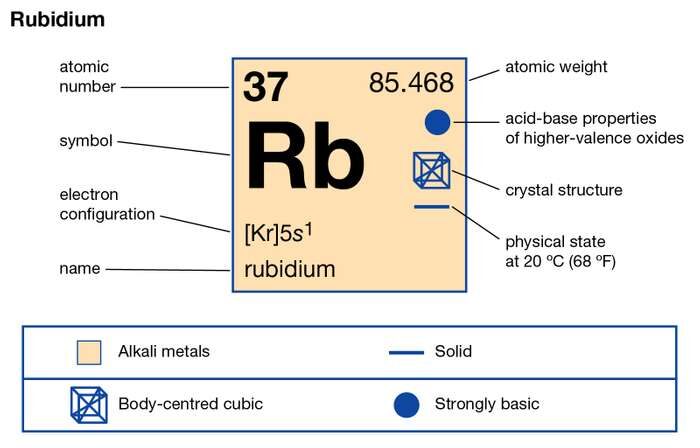
The Group 1 elements in the periodic table are known as the alkali metals. Learn more about these elements including lithium, sodium and potassium.

Potassium electron dot diagram
A step-by-step explanation of how to draw the KNO3 Lewis Dot Structure.For KNO3 we have an ionic compound and we need to take that into account when we draw ... Potassium Dot Diagram. diagram electron dot diagram for potassium template information title electron dot diagram for potassium categories diagram ♦ publised friday january 27th 2017 01 49 18 am what is the electron dot diagram for potassium answers k o h the potassium and hydrogen are in the first group of the periodic table of elements and therefor only need one set of electrons oxygen is ... Therefore the Potassium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. Video: Potassium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.
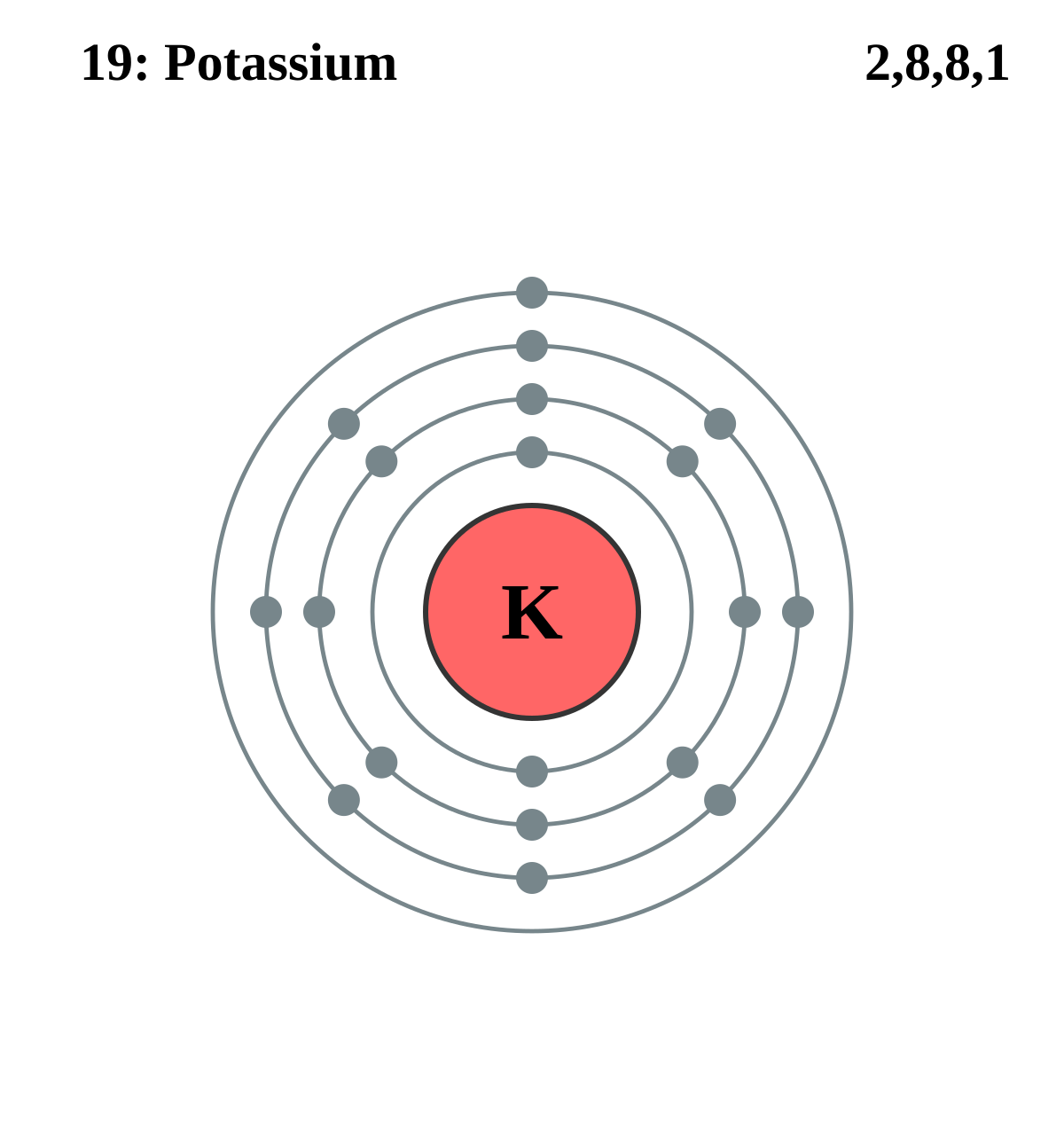
Potassium electron dot diagram. Potassium Explain that potassium has 19 protons and 19 electrons. There are 2 electrons on the first energy level, 8 electrons on the second level, 8 electrons on the third energy level, and 1 on the fourth energy level. Explain that after the third energy level has 8 electrons, the next electron goes into the fourth level. Calcium However, potassium has a single dot or electron in its dot diagram. This diagram shows how much easier it is to lose this lone electron than to find seven to fill the seven empty spaces. When the potassium loses its electron, it becomes positively charged. When chlorine gains the electron, it becomes negatively charged. Opposite charges attract ... Potassium 19 Lewis (Dot) Diagram: Potassium atoms only have 1 valence electron. Example 3: Potassium. Kr Kr Krypton 36 Lewis (Dot) Diagram: Even atoms with more than 20 electrons are easy. Example 4: Krypton Krypton is in group 8A. Krypton atoms have 8 valence electrons. Ra Ra Radium 88 Potassium : As it belongs to s -block , the electronic configuration of potassium is 1s² 2s² 2p⁶ 3s² 3p⁶4s¹ . Since Potassium has 1 electron in its outermost shell , so its electron dot structure involves K with one dot . Is KCl Lewis structure? Despite its few limitations, the initial step towards deciphering chemical bonding is via ...
potassium dot electron structure lewis draw configuration oxygen diagram symbol sans dejavu electrons glyphs does oxide 4th weeks study final . lewis dot structure potassium draw complete guide electronic atom configuration ions atomic . potassium lewis dot structure iodide bonding represents ... Draw electron dot representation for the formation of potassium chloride. MEDIUM. Answer. The electron dot structure for the formation of potassium chloride is given above. Answered By . toppr. How satisfied are you with the answer? This will help us to improve better. answr. Get Instant Solutions, 24x7. Potassium's atomic number is 19. This means that every atom of potassium has 19 protons in its nucleus. In a neutral atom, the number of protons is equal to the number of electrons. So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"4s"^1". The noble gas notation is "[Ar]4s"^1". Since Potassium has 1 electron in its outermost shell , so its electron dot structure involves K with one dot . What is the structure of potassium chloride? The crystal structure of potassium chloride is like that of NaCl. It adopts a face-centered cubic structure. Its lattice constant is roughly 6.3 Å. Crystals cleave easily in three directions.
This study was designed to quantitate and compare the major features of the short-term pharmacokinetics of fluoride--i.e., the plasma (Cp), renal (Cr), and extra-renal (Cer) clearances--in young adult dogs, cats, rabbits, rats, and hamsters.Plasma and urine samples were collected for seven h after the iv administration of fluoride (0.5 mg F/kg). Cp ranged from 3.5 to 8.6 mL/min/kg in the dog ... Lewis Electron Dot Diagram: Lewis electron dot diagram is a diagram which shows the number of valence electron/s that an atom has. The valence electrons are the outermost electrons which means ... Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. Dot structures make it easy to count electrons and they show the number of electrons in each electron shell. Arrow and line diagrams show the spin of electrons and show every orbital. Written configurations require minimal space and show the distribution of electrons between subshells.

Expert Answer Write The Electron Dot Structure For Potassium And Chlorine Show The Formation Of Kcl Brainly Com
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
The electron dot structure : It is representation of valence electrons around the symbol of atoms. Potassium : As it belongs to s -block , the electronic configuration of potassium is 1s² 2s² 2p⁶ 3s² 3p⁶4s¹ .Since Potassium has 1 electron in its outermost shell , so its electron dot structure involves K with one dot.
In an electron dot diagram of potassium There is one dot. In an electron dot diagram of silicon there are four dots. Which element would you expect to be more reactive? silicon.
13.08.2021 · Calcium—Electron Dot Configuration of Calcium is: .Ca. Steps to determine the electron dot diagram. 1) Analyse the total number of valence electrons of every atom in a molecule. 2) In case of anion molecule, add the extra electrons around the element while drawing dot diagram.

10 Fa Write The Electron Dot Structures For Potassium And Chlorine 16 Show The Formation Of Kcl By The Transfer Of Electrons Name The Ions Present In This Compound Kci Atomic Number Of
Dot structures make it easy to count electrons and they show the number of electrons in each electron shell. Arrow and line diagrams show the spin of electrons and show every orbital. Written configurations require minimal space and show the distribution of electrons between subshells.
Electron dot structure. (a) Electrovalent bonding: • Electron dot structure of Electrovalent compounds NaCl, MgCl. 2, CaO. • Characteristic properties of electrovalent compounds state of – existence, melting and boiling points, conductivity (heat and electricity), dissociation in solution and in molten state to be linked with electrolysis. (b) Covalent Bonding: • Electron dot structure ...
The dot-cross diagram is an essential part of explaining how covalent bonding works. A dot-cross diagram consists of nothing but circles, dots, and crosses. The dot represents electron(s) from one atom, while the cross represents electron(s) from the other atom. When one pair of the electron is shared, it means one covalent bond is formed. One covalent bond is represented by dot-cross. Below ...
29.09.2021 · Electron Dot Diagram of CO 2. The valence shell of an oxygen atom includes six electrons. Four of the valence electrons are in lone pairs, meaning that in order to achieve an octet configuration, the oxygen atom must engage in two single bonds or one double bond. Because an O 2 molecule has just two oxygen atoms, the atoms form a double bond, resulting in the Lewis electron dot structure …
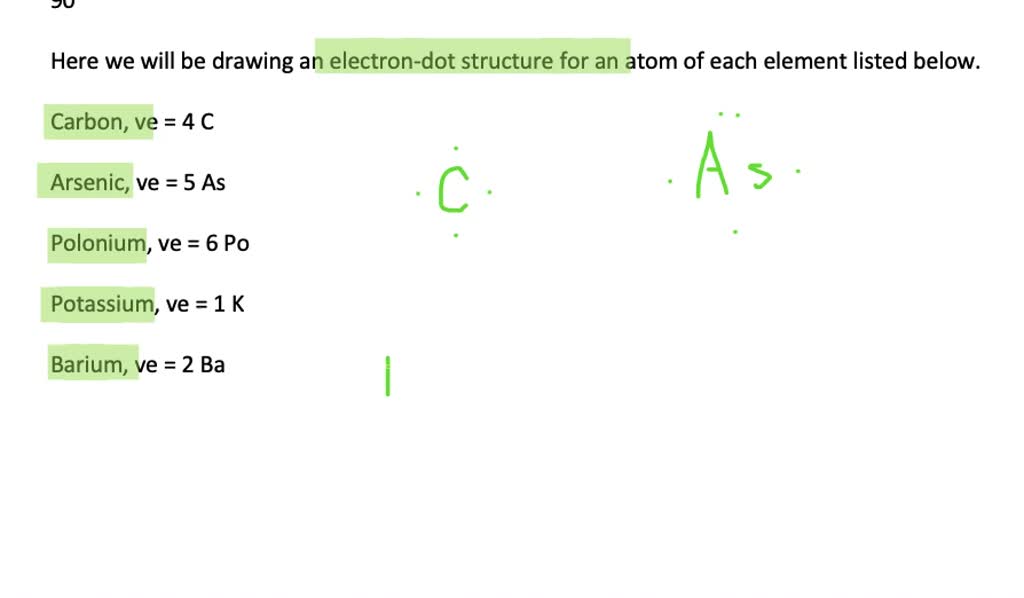
Solved Draw An Electron Dot Structure For An Atom Of Each Element A Carbon B Arsenic C Polonium D Potassium E Barium
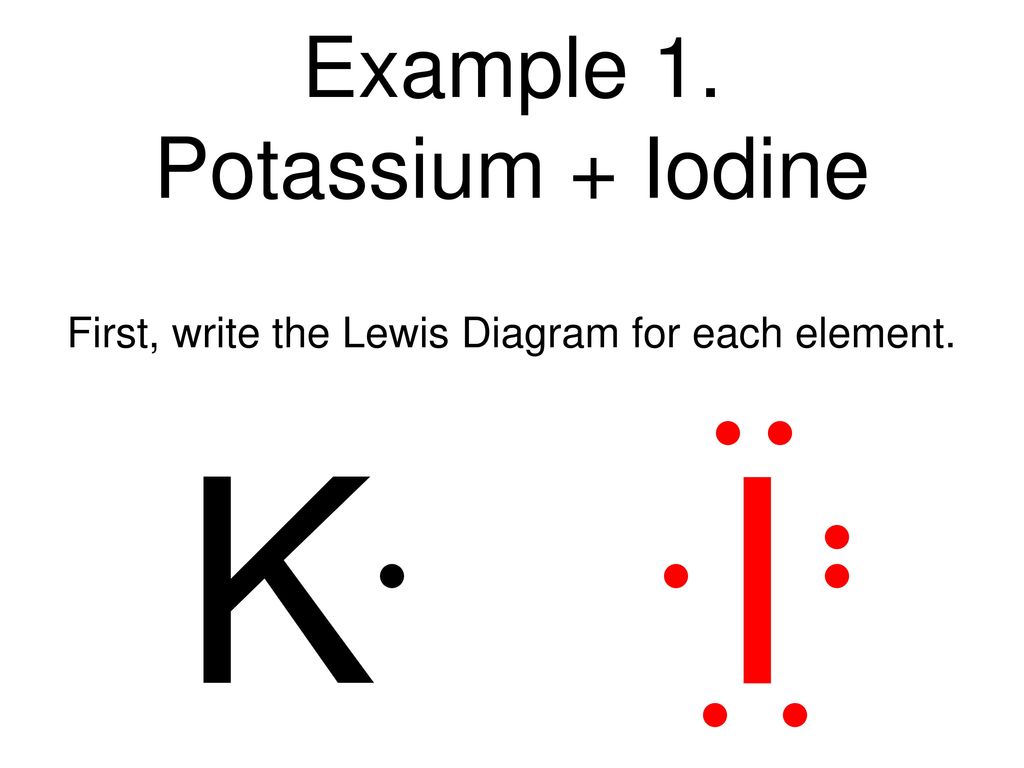
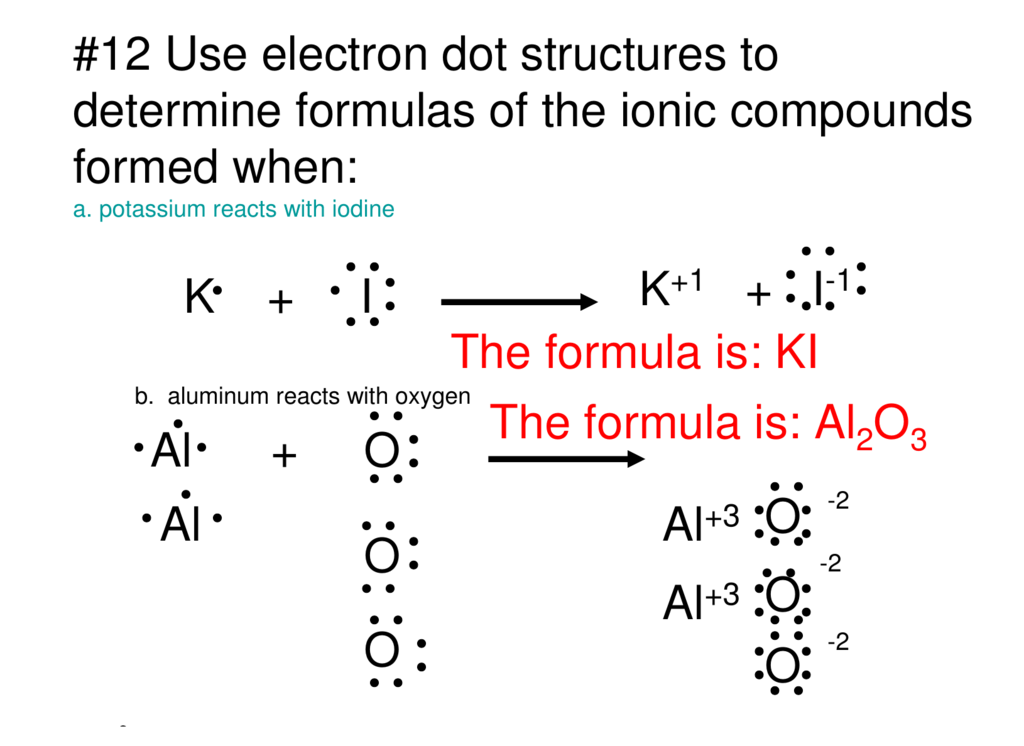
Click here👆to get an answer to your question ️ 2.20 (a) (b) (c) Draw the electron dot structure for Potassium and Chlorine separately. Show the formation of KCl by electron dot structure. Name the ions present in the compound. [Atomic Number K-19, CI-17]
Contributors; Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom.The kernel of the atom, i.e., the nucleus together with the inner electrons, is represented by the chemical symbol, and only the valence electrons are drawn as dots surrounding the chemical symbol.
Answer (1 of 2): Potassium is in the first column, so it has 1 valence electron. Meanwhile, chlorine is in the 7A column, so it has 7 valence electrons. Chlorine will fill its shell by taking potassium's electron that it didn't really want anyway. Now both atoms have complete electron shells. The...

Where Does The Extra Electron Come From In The Lewis Dot Electron Structure Of Say For Example Nitrate Ion Chemistry Stack Exchange
Potassium And Nitrogen Lewis Dot Structure, This is how the ionic bond forms in Potassium Nitride (K3N, File:Lewis dot K svg Wikimedia Commons, How To Draw Electron Dot Structures Green Planet Solar, Hydroxide Wikipedia
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, …
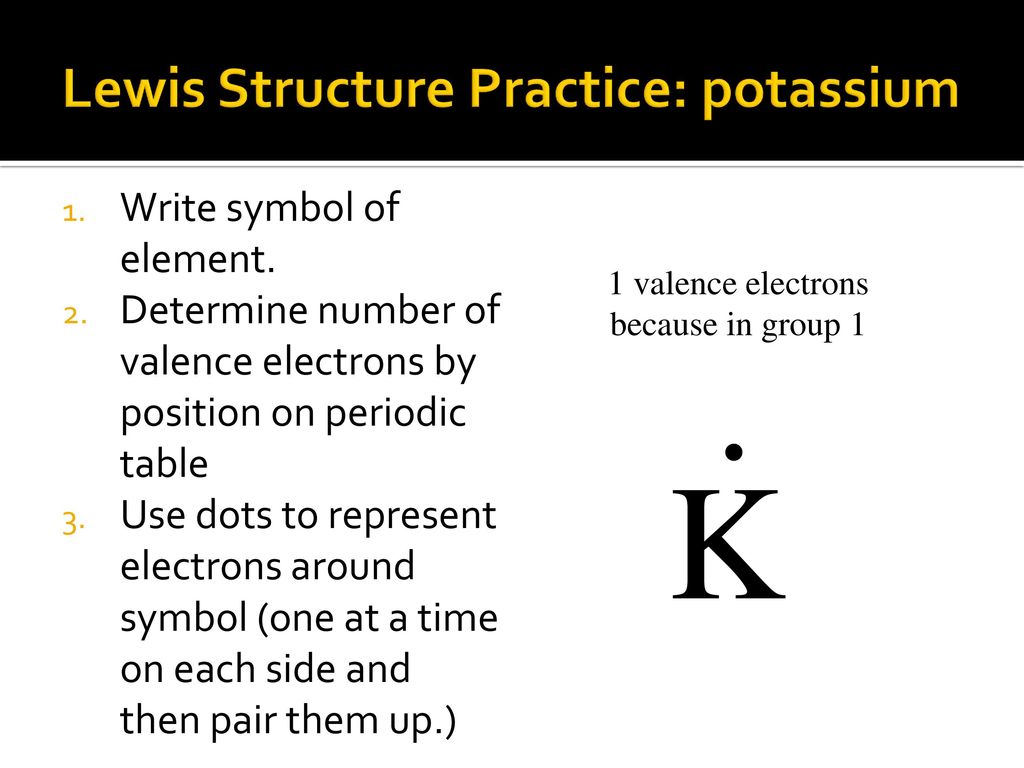
The center of any Lewis Dot Diagram must be the element symbol. In this case it would be "K" for potassium. The electrons have to be placed in a certain order around the element symbol. They are placed in pairs on each side of the element symbol. There has to be a singular electron on each side before a fifth electron can be added to make a pair.
Please find above the Lewis Dot Structure for KCl (Potassium Chloride). As per usual you could replace the one bond with two electrons. In the case for KCl the electronegativity difference between potassium and chloride is so strong (.82 vs. 3.16, respectively) that the bond is considered ionic.
By this electron-dot diagram, you can understand the electron arrangement of individual atoms in a molecule. Also, this diagram can help you to understand how the single pair of electrons can exist inside a molecule. This way, it will be easy to understand the reaction between potassium cation and bromine anion, here is the Lewis dot structure ...
Potassium (1+) is a monoatomic monocation obtained from potassium. It has a role as a human metabolite and a cofactor. It is an alkali metal cation, an elemental potassium, a monovalent inorganic cation and a monoatomic monocation. Potassium is the major cation (positive ion) inside animal cells, while sodium is the major cation outside animal ...
A Write The Electron Dot Structures For Potassium And Chlorine B Show The Formation Of Kcl By The Transfer Of Electrons Sarthaks Econnect Largest Online Education Community
Start studying Electron Dot Diagrams. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Potassium : As it belongs to s -block , the electronic configuration of potassium is 1s² 2s² 2p⁶ 3s² 3p⁶4s¹ .Since Potassium has 1 electron in its outermost shell , so its electron dot structure involves K with one dot . Chlorine : Chlorine belongs to p-block so the electronic configuration of Cl is 1s² 2s² 2p⁶ 3s² 3p⁵ .
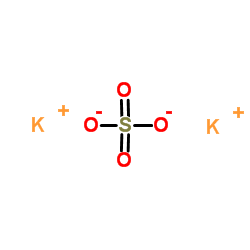
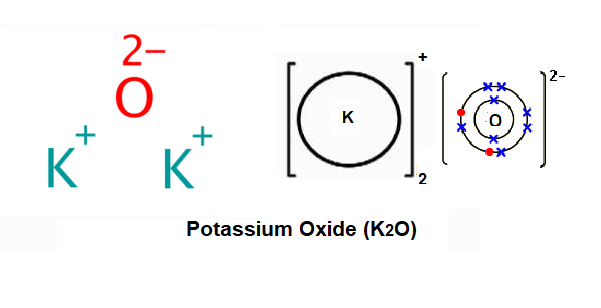
Potassium oxide is an ionic compound formed by combining potassium and oxygen. It carries the chemical formula K2O. Potassium cannot be found free because it is too reactive. It has valency +1 and combines readily with oxygen atoms forming K 2 O. The oxide, K 2 O, is obtained as a grey crystalline substance when potassium is oxidized; potassium ...
Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 …
Therefore the Potassium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. Video: Potassium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.
Potassium Dot Diagram. diagram electron dot diagram for potassium template information title electron dot diagram for potassium categories diagram ♦ publised friday january 27th 2017 01 49 18 am what is the electron dot diagram for potassium answers k o h the potassium and hydrogen are in the first group of the periodic table of elements and therefor only need one set of electrons oxygen is ...
A step-by-step explanation of how to draw the KNO3 Lewis Dot Structure.For KNO3 we have an ionic compound and we need to take that into account when we draw ...







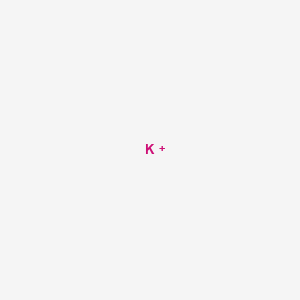


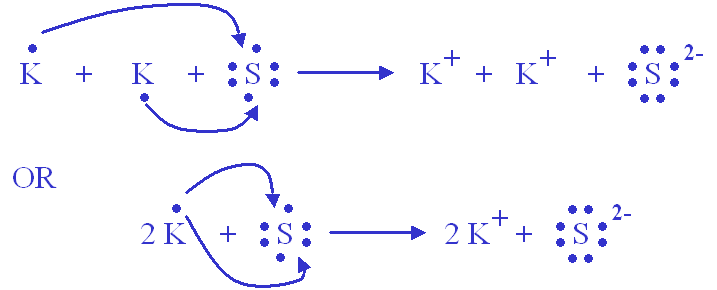







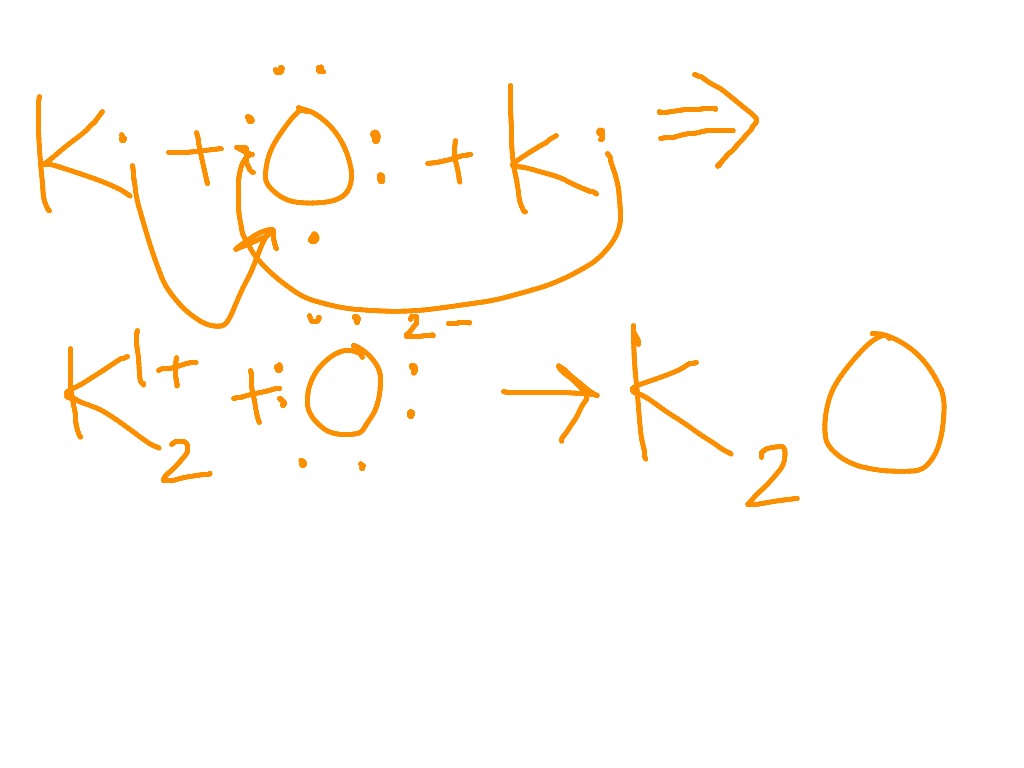

Comments
Post a Comment