40 sulfur lewis dot diagram
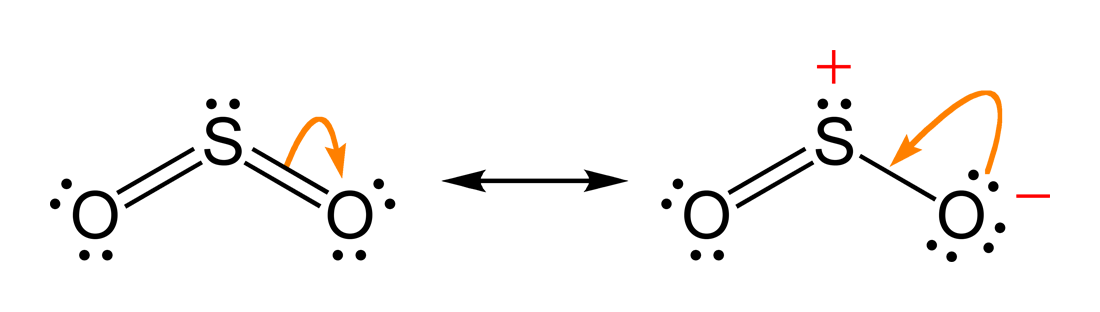
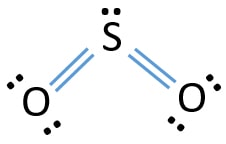
One point is earned for the molecular geometry consistent with the Lewis diagram in part (a). (c) In the SO 2 molecule, both of the bonds between sulfur and oxygen have the same length. Explain this observation, supporting your explanation by drawing in the box below a Lewis electron-dot diagram (or diagrams) for the SO 2 molecule. To sketch the SO2 Lewis structure by following these instructions: Step-1: SO2 Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of SO2 for counting valence electrons around the terminal oxygen atoms. Step-3: Lewis dot Structure for SO2 generated from step-1 and step-2.
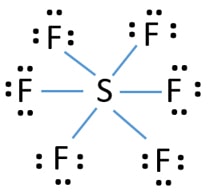
SF6 is a colorless and odorless gas that is non-combustible and non-flammable in nature. The central atom here is sulfur bonded with 6 fluorine atoms. Lewis dot structure has 6 sigma bonds and rests lone pairs on fluorine. The hybridization of SF6 is sp3d2. SF6 has octahedral molecular geometry and is non-polar in nature.

Sulfur lewis dot diagram
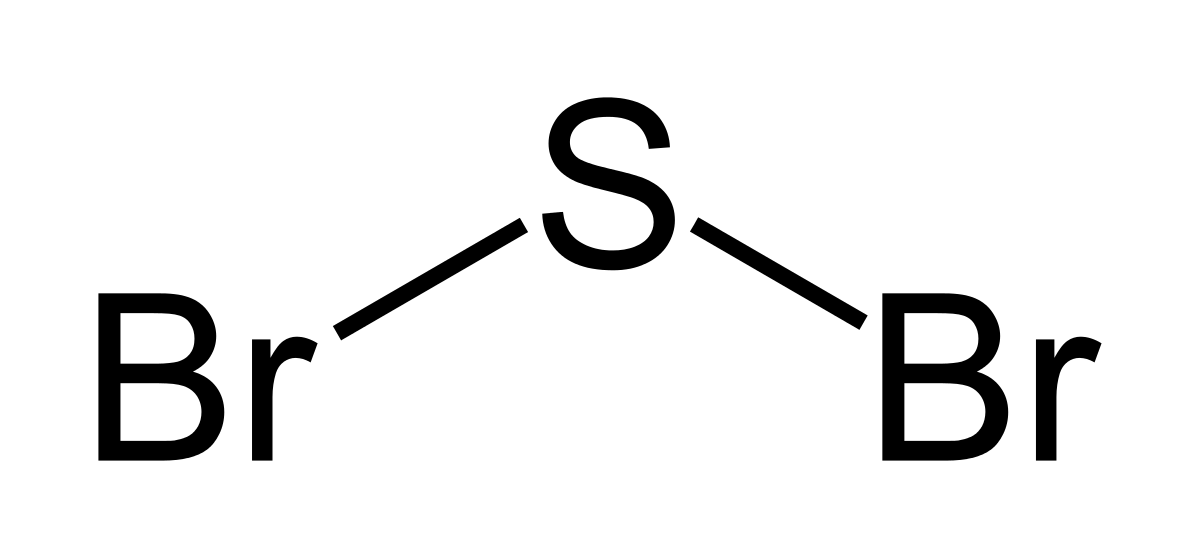
SBr2 Lewis structure is made up of two atoms, sulfur, and bromine, the sulfur is in the central position and bromine atoms are in the surrounding position. The lewis structure of SBr2 contains 16 nonbonding electrons and 4 bonding electrons. The lewis structure of SBr2 is similar to the SCl2 and it is very easy to draw. Here let's see how to ... Moreover, what is the Lewis dot structure for sulfur? Now let us try Lewis dot structure of Sulfide ion ( S 2-). Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom. Sulfur dioxide (SO 2) Lewis Structure, Hybridization. Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. We will construct the lewis structure of SO 2 molecule by following VSEPR theory rules and considering stability of intermediate structures. After obtaining the lewis structure of SO 2, we can determine the hybridization of atoms.
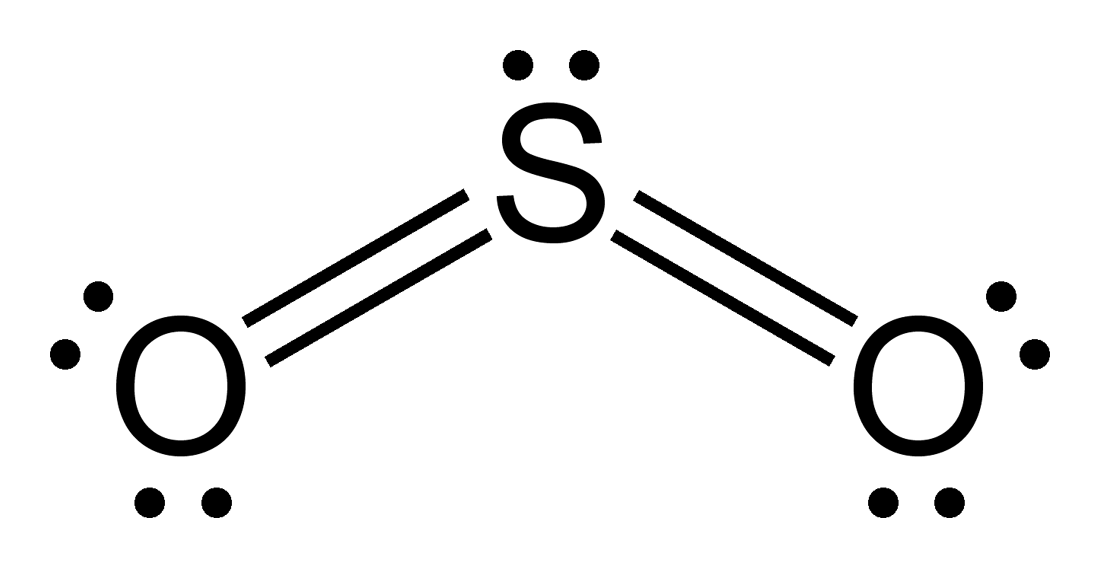
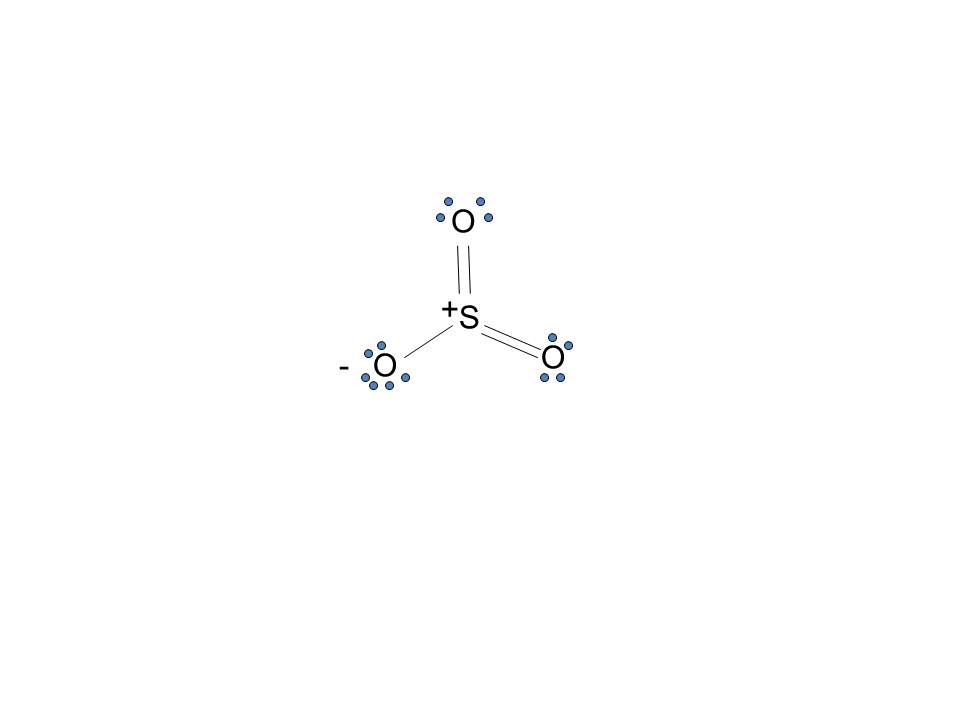
Sulfur lewis dot diagram. SO3 - Sulfur Trioxide: First draw the Lewis dot structure: Electron geometry: trigonal planar. trigonal planar e. square planar c. Aug 25, 2021 · Lewis structure is a representation of all the bonds and lone pairs of different atoms that a compound has. Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. It has a chemical formula of SF 2 and can be generated by the reaction of Sulphur Dioxide and Potassium Fluoride or Mercury Fluoride. In this blog post, we will look at the Lewis dot structure of SF 2, its molecular geometry and shape. The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur. In the SO3 Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the SO3 Lewis structure, with all three oxygen atoms arranged in trigonal planar geometry. Add valence electrons around the oxygen atom, as given in the figure. Step-3: Lewis dot Structure for SO3 generated from step-1 and ...
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2. Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen. Sulfur being the less electronegative atom than chlorine atom is placed at the center in lewis's diagram and chlorine is spaced evenly around it. There is two lone pair present on the central atom and this central atom attached to two bonded pair in SCl2 lewis structure. Steps to draw electron dot structure or lewis structure of SCl2 The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms. It is known as a formula written as SO2. Here we will provide an explanation of SO2 molecular geometry, SO2 electron geometry, SO2 bond angle, and SO2 Lewis structure.
Lewis electron dot diagram. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol ... SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. This compound is generally identified as being a colorless gas. The molecular weight of this compound is calculated to be 108.6 g/mol. SF4's boiling and melting points are -38 ... A step-by-step explanation of how to draw the SI2 Lewis Dot Structure.For the SI2 structure use the periodic table to find the total number of valence electr... Lewis Dot Diagram For So4 2. Simple procedure for drawing covalent Lewis structures - Lewis dot of the sulfate ion SO, best lewis structure for so, electron bonding. Viewing Notes: The Lewis structure for SO is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis.
A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ...
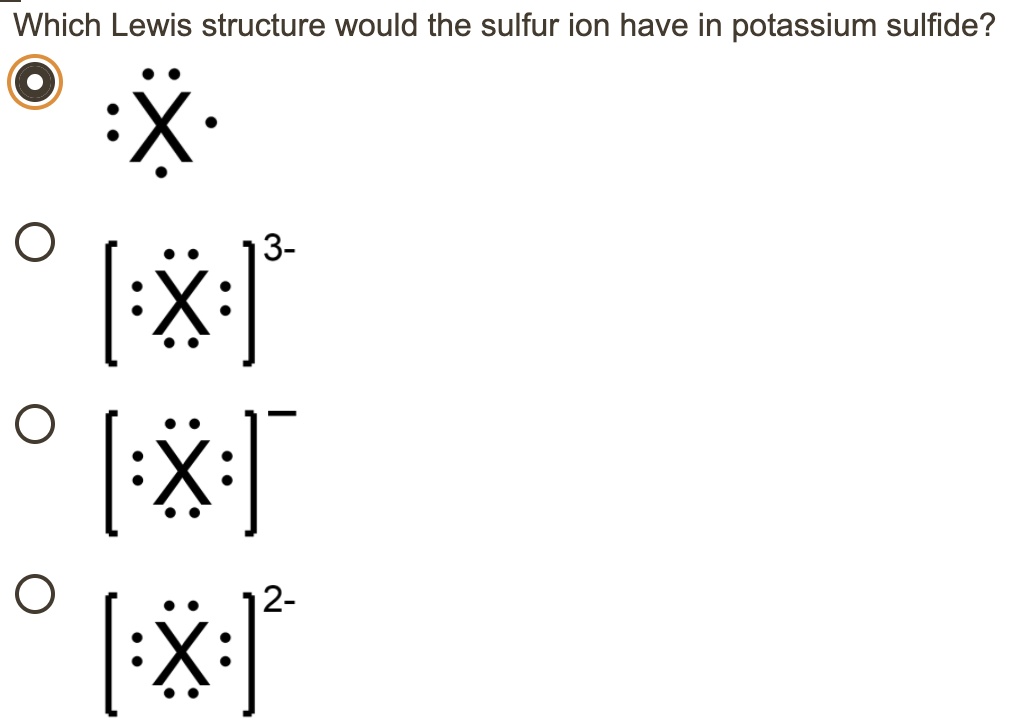
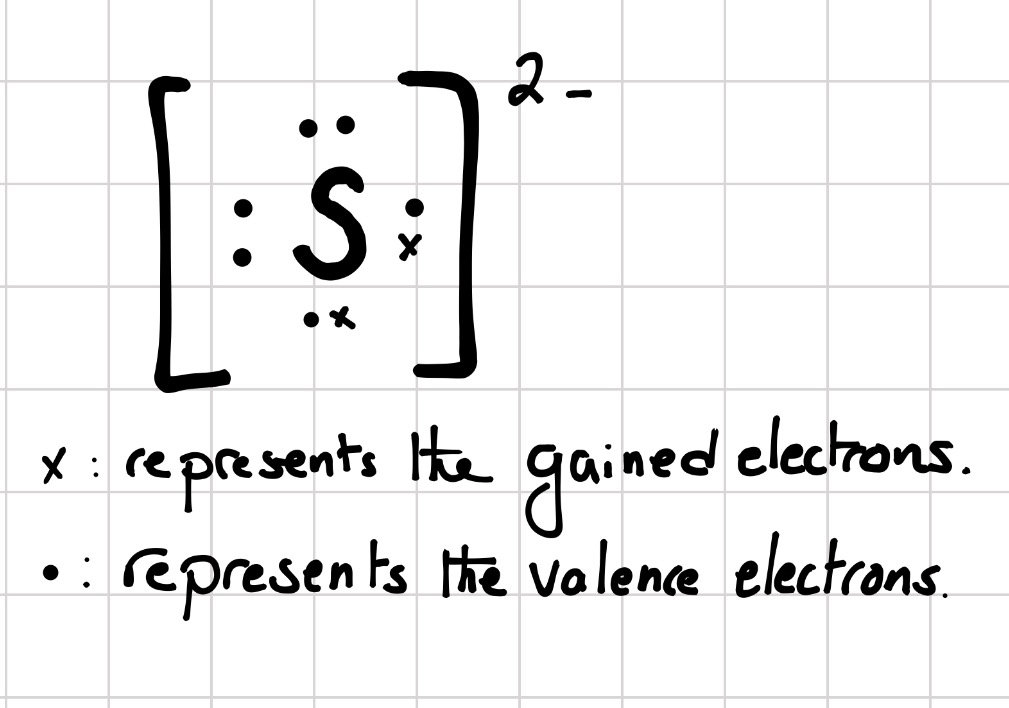
What is the Lewis dot structure for sulfur? Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom. For atoms and monoatomic ions, step one is sufficient to get the correct Lewis structure. ...
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access to the . Here are the steps I follow when drawing a Lewis structure.
A neutral sulfur atom has 6 electrons, regardless of the isotope. The lewis dot structure would have a pair of dots on two sides of the symbol S, and a single dot on the other two sides, for a ...
Xenon has 8 dot s (4 pairs of dot s) around the letters Xe. for XeF4. Step-by-step tutorial for drawing the Lewis Structure for XeF4. for the molecule. Remember that Xenon can have more than 8 valence electrons. Comprehensive in for mation for the element Xenon - Xe is provided by this page including scores of Atomic Structure of Xenon Electron Dot...A step-by-step explanation of how to draw ...
Lewis dot structure of Nitride ion. Now let us try Lewis dot structure of Sulfide ion ( S 2-).Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). [Ne]4s 2 4p 6. Valence electrons are 8 (2 in 3s and 6 in 3p) Lewis dot structure of sulfide ion

Draw The Lewis Structure For The Sulfur Trioxide So 3 Molecule Be Sure To Include All Resonance Structures That Satisfy The Octet Rule Study Com
Similarly, it is asked, what is the Lewis dot structure for sulfur? Now let us try Lewis dot structure of Sulfide ion ( S 2-). Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom.
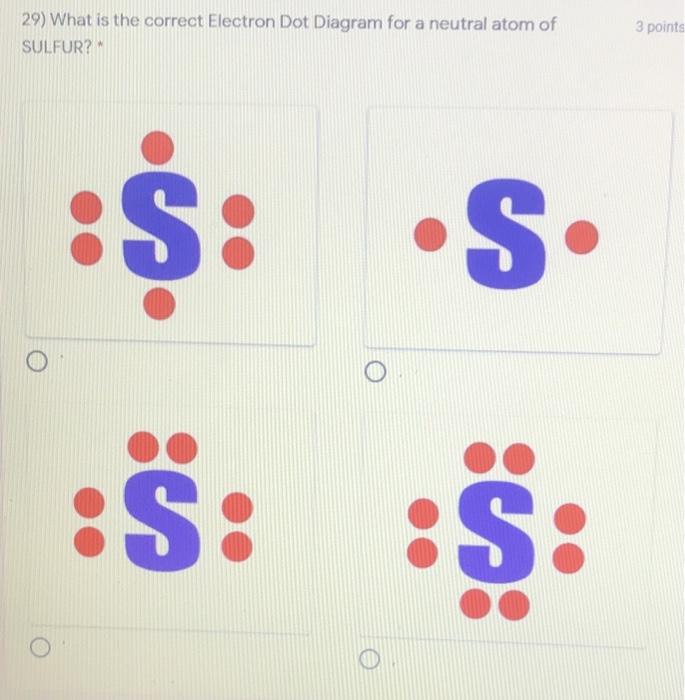
What is the correct electron dot diagram for a neutral atom of SULFUR ? Answer : Surfum (s) electronic configuration, is? 252 286 352 3p 4 valence shell for Sulfur 352 384 The ne fone, electron dot diagram of a neutral sulfur is. :5: Hottest videos.

Carbonyl Sulfide Has The Chemical Formula Cos Carbon Has Four Valence Electrons And Oxygen And Brainly Com
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Sulfur dioxide (SO 2) Lewis Structure, Hybridization. Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. We will construct the lewis structure of SO 2 molecule by following VSEPR theory rules and considering stability of intermediate structures. After obtaining the lewis structure of SO 2, we can determine the hybridization of atoms.
Moreover, what is the Lewis dot structure for sulfur? Now let us try Lewis dot structure of Sulfide ion ( S 2-). Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom.
SBr2 Lewis structure is made up of two atoms, sulfur, and bromine, the sulfur is in the central position and bromine atoms are in the surrounding position. The lewis structure of SBr2 contains 16 nonbonding electrons and 4 bonding electrons. The lewis structure of SBr2 is similar to the SCl2 and it is very easy to draw. Here let's see how to ...
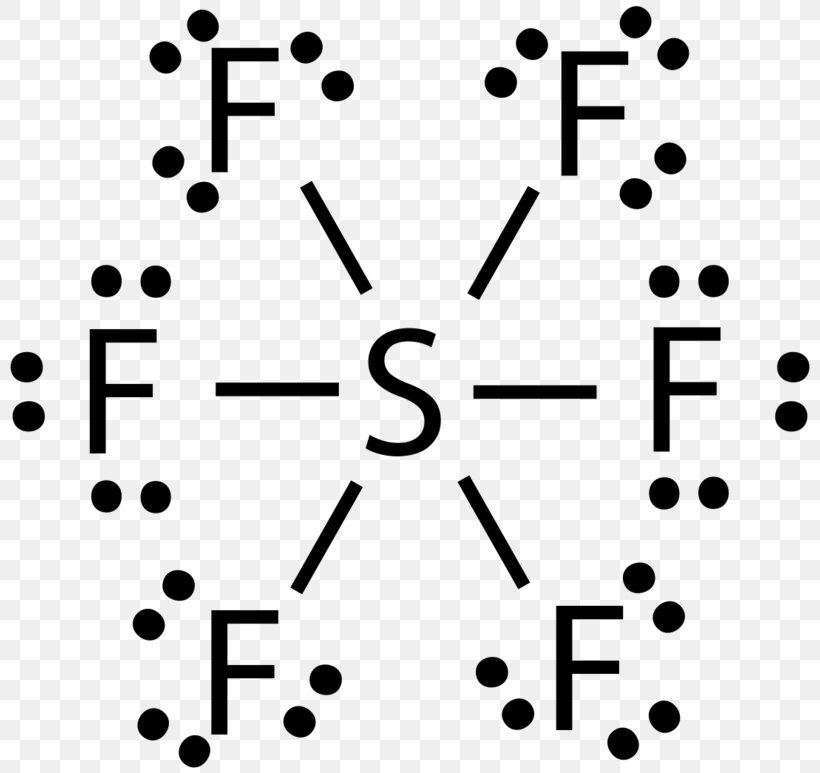
Lewis Structure Sulfur Hexafluoride Lewis Pair Vsepr Theory Sulfur Tetrafluoride Png 800x773px Watercolor Cartoon Flower Frame



















Comments
Post a Comment