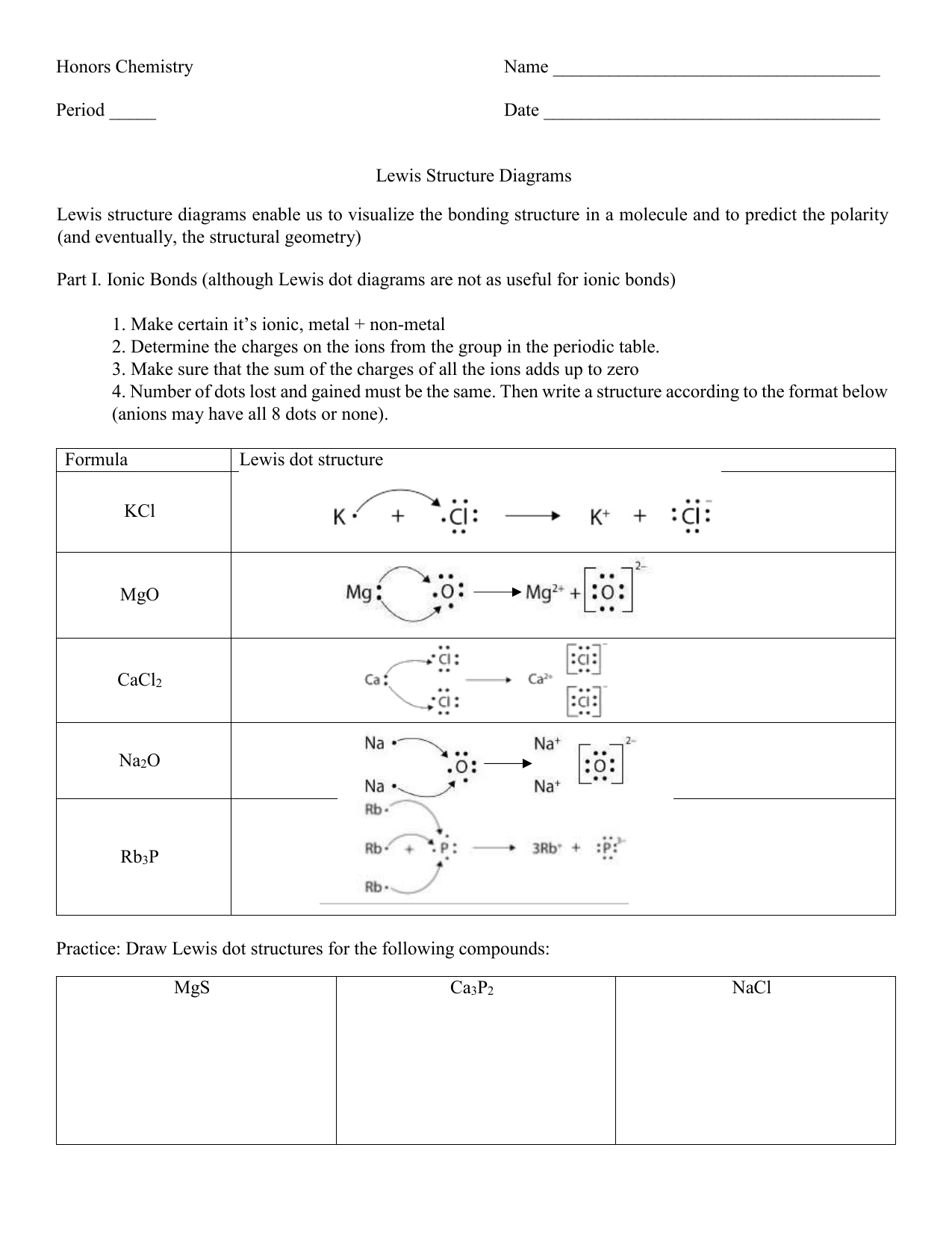
38 lewis dot diagram ch4
They also do not produce any lewis dot structure. Is CH4O a resonance? These two structures are called resonance structures, and molecules such as benzene, which have two or more resonance structures, are said to exhibit resonance. Carbon dioxide, CO2, which is linear, is a nonpolar molecule; methane, CH4, which is tetrahedral, is also nonpolar. The dot structure of Na+1 is Na+1 . The dot structure of O-2 is O-2. Note that Na is in group 1 and should lose 1 electron while O is in group 6 and should gain 2 electrons. ionic compounds Make certain it's ionic: one atom must be from groups 1-3, the other from groups 4-7 (including H).
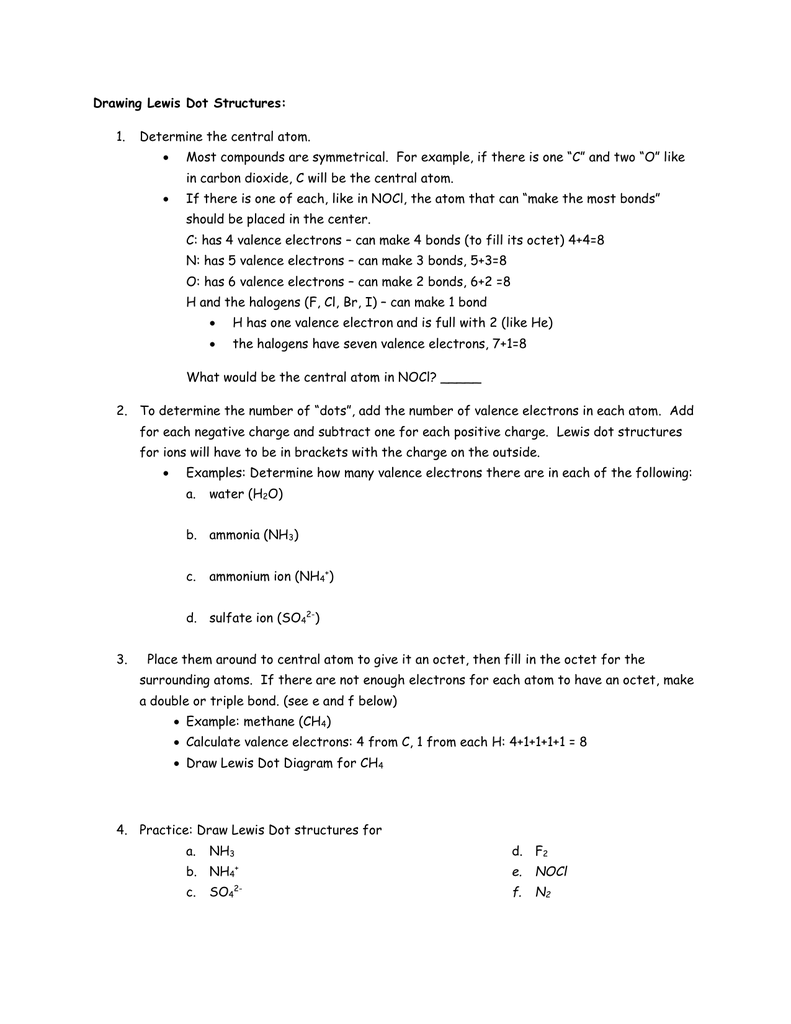
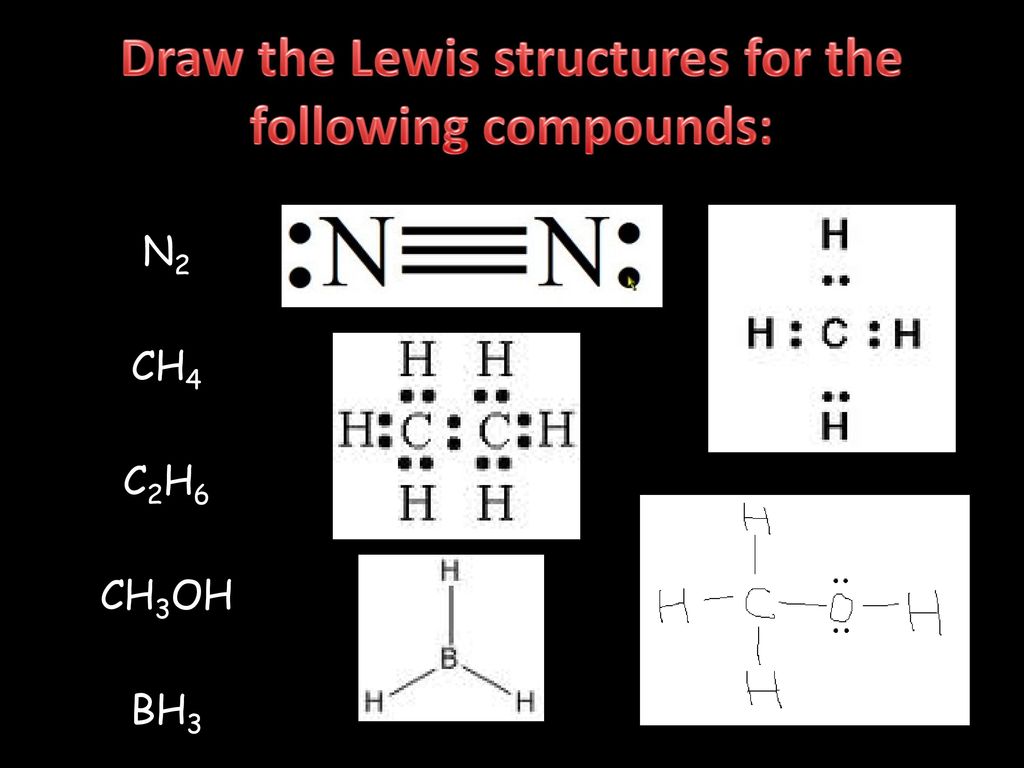
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).

Lewis dot diagram ch4
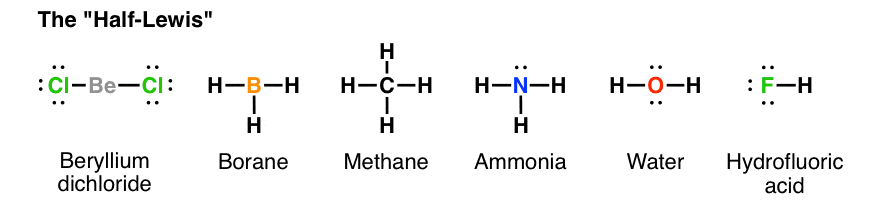
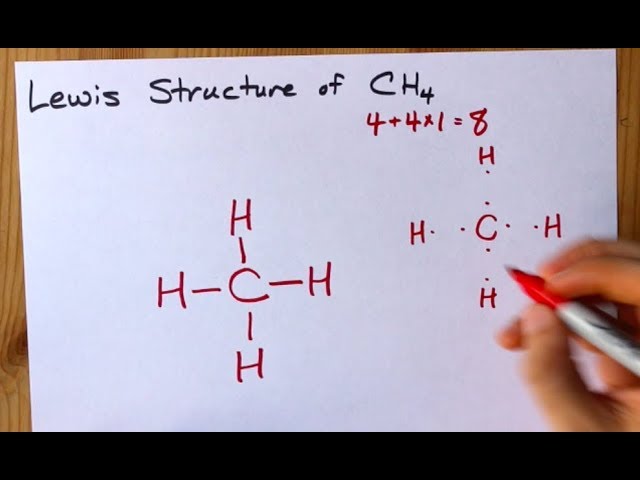
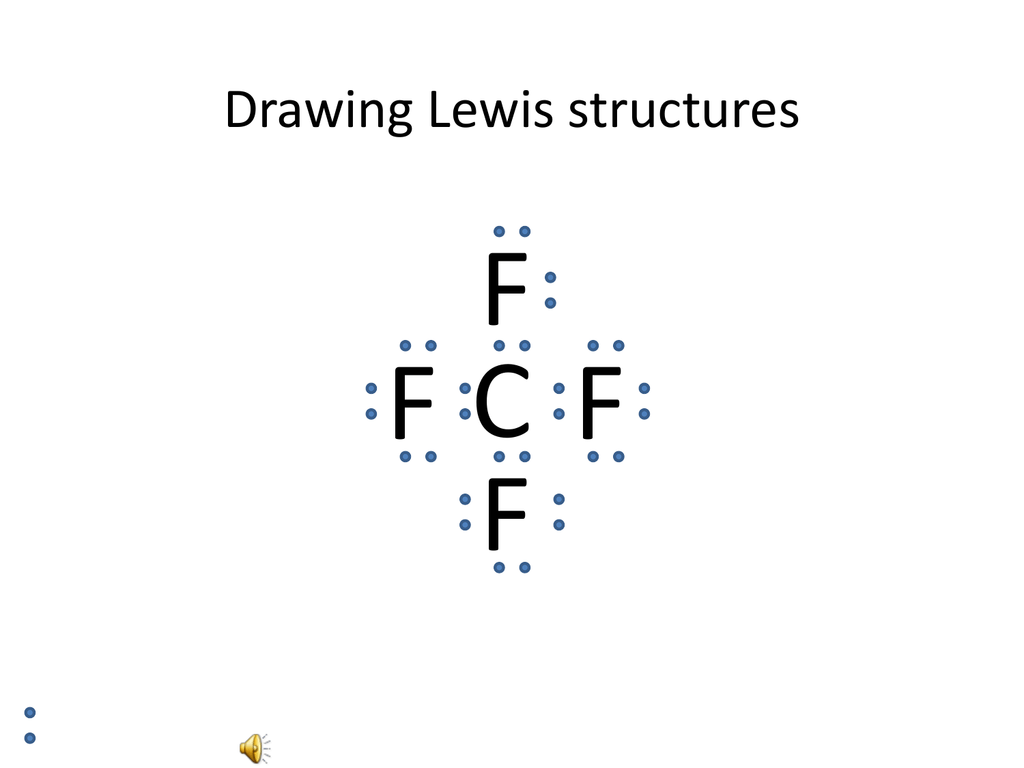
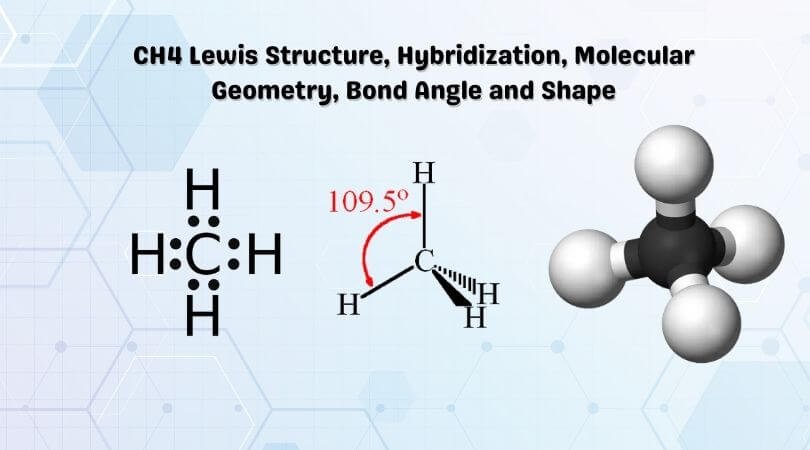
The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3. A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use the periodic table to find the total number of vale... CH4 Lewis Structure. Answer: CH4 Lewis structure (Methane electron dot structure) is that type of diagram where we show the total 8 valence electrons of CH4 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ].
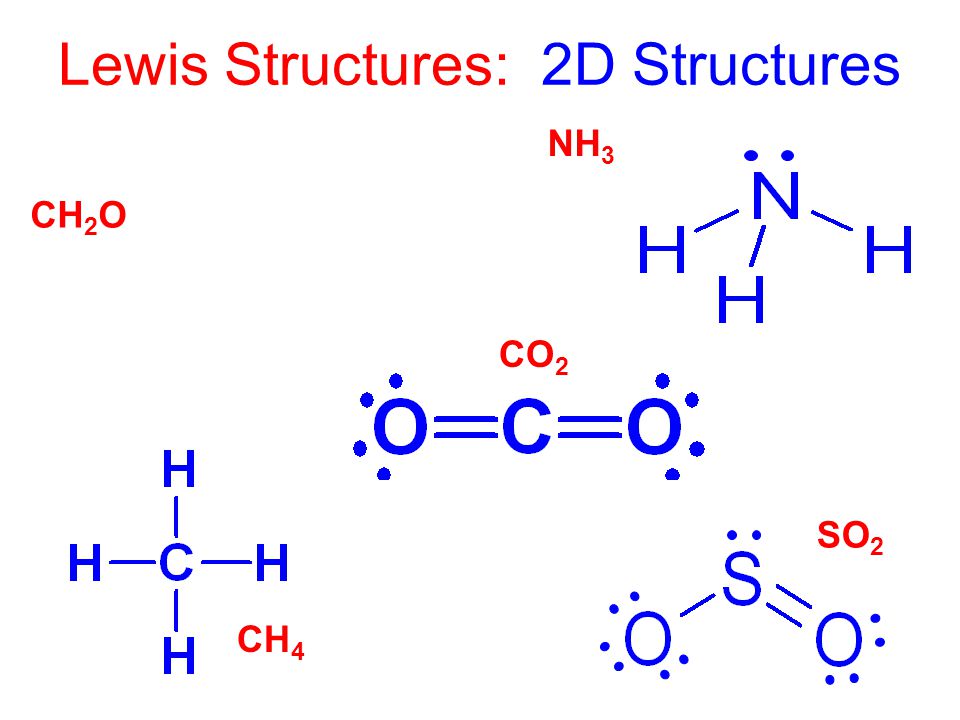
Lewis dot diagram ch4. Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. Dr. B. explains how to draw the Lewis dot structure for CH 4 (methane). The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure. This is because they can share a maximum of two electrons. The quiz will present you with different compounds and then ask you to identify the correct Lewis dot diagram. It will also ask you to identify the various conventions related to Lewis dot ... Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. Source: image1.slideserve.com. I may be wrong, but i think that the lewis dot structure of ch4o consists of the carbon atom being bonded to three of the hydrogen atoms, and the oxygen atom.
Lewis Dot Structure for CH4 How to create a Lewis Dot Structure for CH4 # 2 Find the number of “octet” electrons for the molecule. C: 8 octet electrons x 1. How to draw the Lewis structure of methane, CH4 By José @ Periodic table with names diagramweb.net But seriously, you have an electron pair between the C and each of the H's in the Lewis diagram a ala Why is that the correct diagram ... According to the lewis dot structure of CH4, the Carbon central atom doesn’t contain any lone pair but is attached to the four hydrogen atoms with the help of four bonded pairs. Summary. The total valence electron available for drawing the Methane (CH4) lewis structure is 8. The steric number of the central atoms in methane is 4 that ensures that it has an Sp 3 hybridization. CH4 is a ... Lewis Structures for CH4. Step-by-step tutorial for drawing the Lewis Structure for CH4. The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the . I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to two (this is why hydrogen only needs two).The covalent bonds between the C and the H are similar to the ones formed between two Hs ...
Lewis Dot Structure for CH4 #2 Find the number of “octet” electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . We show two ways to draw the CH4 Lewis structure, methane. We also have This info can then be used to determine the Lewis Dot Structure. Find an answer to your question draw the electron dot structure of CH4.Drawing the Lewis ... What are the steps in drawing a Lewis structure? Follow these simple steps to correctly draw a Lewis dot structure: Add up the total number of valence electrons found in the entire compound. Draw the simple structure (skeleton structure) of the compound by connecting everything with single bonds only. Add electrons to all the noncentral atoms. Lewis Dot Structure for CH4 #2 Find the number of “octet” electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . Find an answer to your question draw the electron dot structure of CH4. The structure of nitrogen is N≡N, showing that it has three shared pairs of electrons. Oxygen and nitrogen with their formulas, structures and dot and ... atoms. We do this by forming what are called Lewis diagrams. In Lewis diagrams the atoms are shown by writing the atomic symbol surrounded by one dot for each of the valence electrons. In a covalently bound molecule the dots are arranged in pairs, with the bound pairs placed between the atoms which they connect
Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
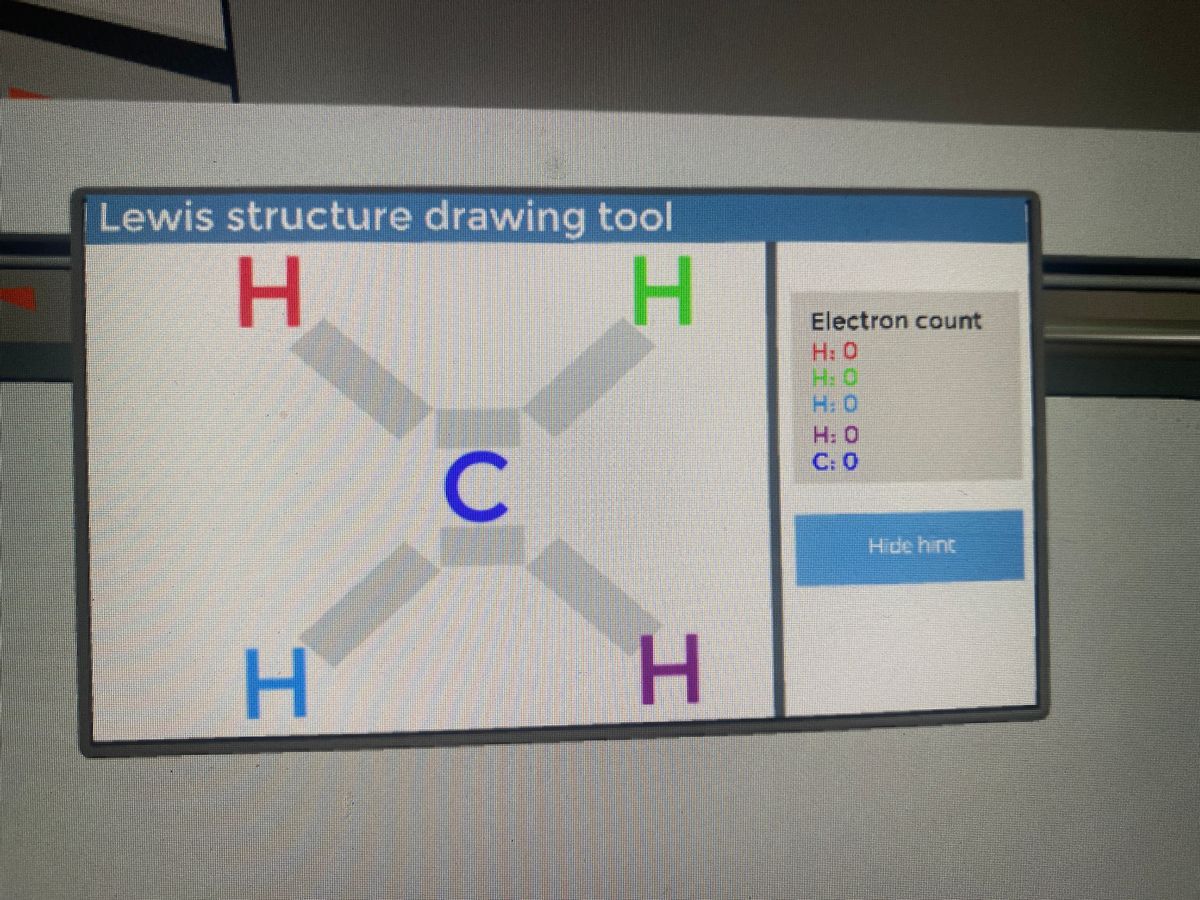
In electron dot structure we represent the valence electron of the element. Thus, Carbon has 4 electrons in its electron dot structure and hydrogen has one. They share electrons to form a C − H single bond.
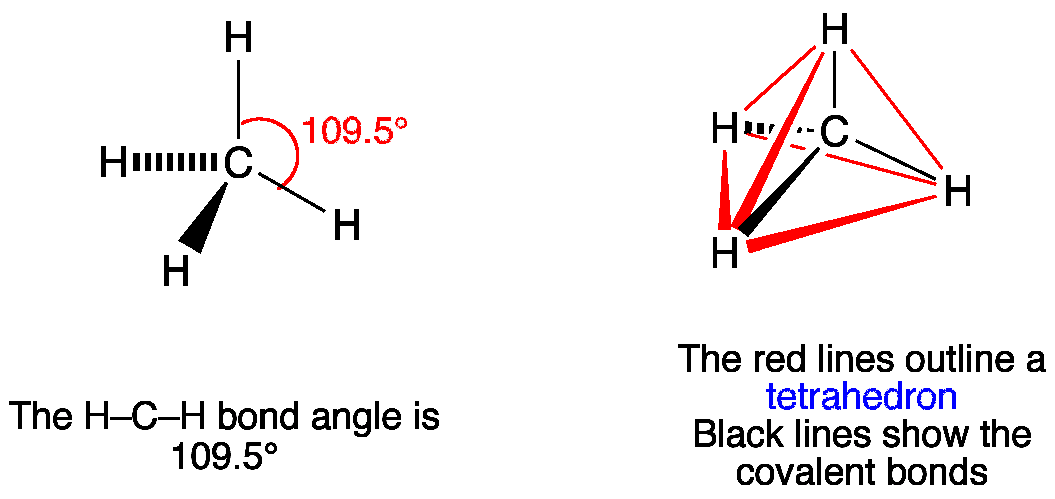
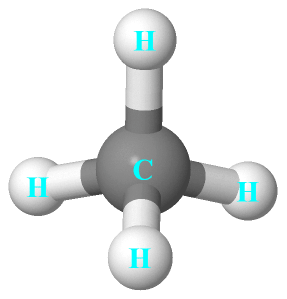
CH4 Lewis Structure, Hybridization, Molecular Geometry, Bond Angle and Shape. Methane is one of the simple organic molecules, given its straightforward structure. It has the chemical formula of CH4 and comprises one carbon atom forming bonds with four hydrogen atoms. The compound is one of the main constituents of natural gas.
Answer: I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the middle is the one Carbon, and the four yellow ones are Hydrogens. Now I have drawn the Valence Electrons. These are the blue dots next to the...
I quickly take you through how to draw the Lewis Structure of methane, CH4. I also go over hybridization, shape and bond angle.
Draw Lewis dot diagram for the following. Methane (CH4) ... Concept: Kossel and Lewis Approach to Chemical Bonding. Report Error
Answer to: Draw the Lewis dot structure for CH4 and provide the following information. a. number of bond pairs b. number of lone pairs c. molecular...
Lewis Dot Structure for CH4 (Methane) Properties of methane are described by Lewis Structure as cheaper natural gas than electricity. Methane, or CH4, is a natural gas that is relatively plentiful on earth, making it an environmentally effective source.
How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step. Firstly, look for the total number of valence electrons required by a ...
Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4.
Answer: I would be lazy and look it up on the internet. But seriously, you have an electron pair between the C and each of the H's in the Lewis diagram a ala Why is that the correct diagram, you ask? First, each Hydrogen has only one electron to donate or share, and remember that Hydrogen's fil...
CH4 Lewis Structure. Answer: CH4 Lewis structure (Methane electron dot structure) is that type of diagram where we show the total 8 valence electrons of CH4 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ].
A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use the periodic table to find the total number of vale...
The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.





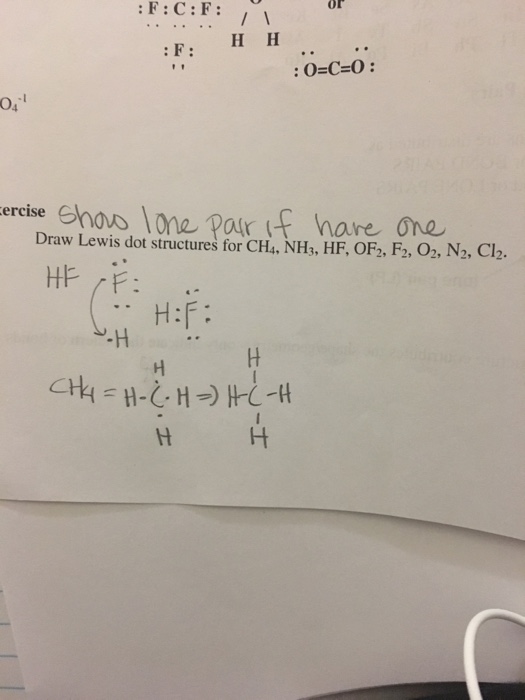















Comments
Post a Comment