38 nacl electron dot diagram
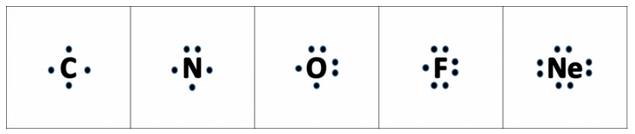
Draw Orbit Structure Diagram of Sodium Chloride (Nacl) CISCE ICSE Class 9. Question Papers 10. Textbook Solutions 19257. Important Solutions 6. Question Bank Solutions 14520. Concept Notes & Videos 431. Syllabus. Advertisement Remove all ads. Draw Orbit Structure Diagram of Sodium Chloride (Nacl) - Chemistry ... The Lewis structure contains the element symbol with dots representing electrons. The only electrons shown are those on the outer energy level or valence electrons. The electrons are placed around the element symbol, one at a time, clockwise or counterclockwise, and then grouped in pairs as more electrons are added.
Sodium Chloride is a metal halide composed of sodium and chloride with sodium and chloride replacement capabilities. When depleted in the body, sodium must be replaced in order to maintain intracellular osmolarity, nerve conduction, muscle contraction and normal renal function.

Nacl electron dot diagram
A step-by-step explanation of how to draw the NaCl Lewis Dot Structure (Sodium chloride).For NaCl we have an ionic compound and we need to take that into acc... The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE. NaCl + NH3 + CO2 + H2O ® NaHCO3 + NH4Cl 15)In the space draw a Lewis electron-dot diagram for the reactant containing nitrogen in the equation. 16)Explain, in terms of electronegativity difference, why the bond between hydrogen and oxygen in a water molecule is more polar than the bond between hydrogen and nitrogen in an ammonia molecule.
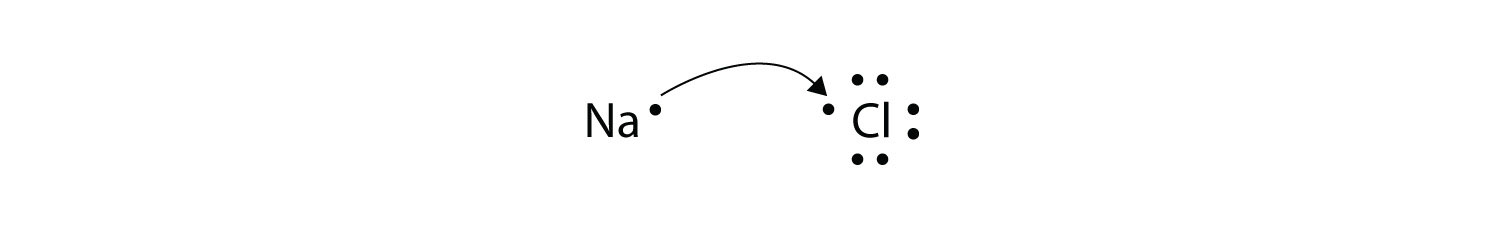
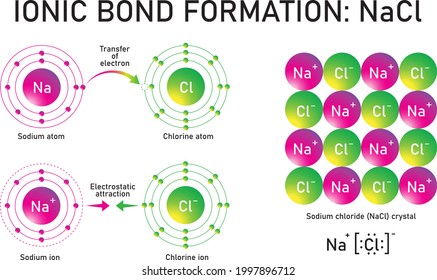
Nacl electron dot diagram. Sodium Electron Dot Diagram Sodium Chloride Dot Diagram Wiring Diagram Review. Sodium Electron Dot Diagram Hydrogen Chloride Electron Dot Diagram For Hydrogen Chloride. Sodium Electron Dot Diagram A Lewis Dot Diagram Is An Easy Way To Represent An Atoms Valence. NaCl lewis structure is unique and very interesting to draw because it is an ionic compound formed from metal(Na+) and nonmetal(Cl-). The lewis dot structure of NaCl contains one positive charge on sodium metal and one negative charge on chlorine nonmetal. Cr and O. Which statement is true about the compound ammonium sulfate [ (NH4)2SO4]? -It is a mixture of three polyatomic ammonium ions and a polyatomic sulfate ion. -It is a salt of two polyatomic ammonium ions and one polyatomic sulfate ion. -It is a salt of monatomic nitrogen, hydrogen, sulfur, and oxygen ions. The formation of sodium chloride can be shown more clearly with the help of a diagram as shown below, From the diagram, we can say that one electron is transferred from each sodium atom to each chloride atom resulting in the formation of oppositely charged sodium ions and chloride ions.
Sodium Chloride is the chemical name of NaCl. The NaCl Molecular Weight (Sodium Chloride) is 58.44 g/mol. Visit BYJU'S to understand the properties, structure, and uses of Sodium Chloride (NaCl) explained by India's best teachers. Nov 19, · A step-by-step explanation of how to draw the NaCl Lewis Dot Structure. For the NaCl Lewis structure, calculate the total number of valence electrons for the . Lewis Dot Formulas of Atoms zLewis dot formulas or Lewis dot representations are a convenient bookkeeping method for tracking valence electrons. Formation of NaCl The following electron dot diagram shows the formation of NaCl. Formation of MgF 2 The following electron dot diagram shows the formation of MgF 2. Notice that instead of drawing electron dot diagrams for 2 F-, the number "2" has been placed in front of one of the F-. A step-by-step explanation of how to draw the NaCl Lewis Dot Structure.For the NaCl Lewis structure, calculate the total number of valence electrons for the ...
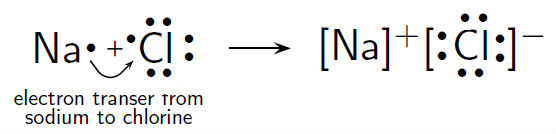
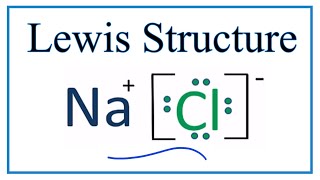
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element. Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. The Lewis dot structure of NaCl consists of a chloride ion surrounded by eight electron dots (four pairs) and a sodium ion bonded to that chlorine ion. Typically, ionic Lewis dot structures include the ionic charge, so the Na ion is labeled +1 and Cl is labeled -1. The Lewis dot structure of ionic bonds such as NaCl is formed by looking at both ... (2) Electron dot structural diagram (3) Atomic or orbital structural diagram the formation of the following. (a) Sodium chloride (b) Calcium oxide (c) Magnesium chloride. (at. nos. Na = 11, Cl = 17, Ca = 20, O = 8, Mg = 12)
View solution. >. The skeletal structure of C H 3 C O O H as shown above is correct but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid. Medium. View solution. >. Chlorine is represented by the Lewis structure (image). The atomic number of an atom that will give an identical electron-dot arrangement as ...
The lewis structure for the salt nacl shows two ions which have their now outer shells of electrons filled with a complete octet. For the nacl lewis structure calculate the total number of valence electrons for the nacl molecule. The lewis dot structure is a diagram to show the bonding between the atoms of a molecule and pairs of electrons that ...
The Lewis dot structure of NaCl consists of a chloride ion surrounded by eight electron dots (four pairs) and a sodium ion bonded to that chlorine ion. Thus, to determine the oxidation number of the two atoms, we count the electrons It is important to keep the formal charges as low as possible. and hence several thousands times slower than electrons).
Toppr: Better learning for better results

34 Lewis Dot Structure Of Nacl How To Draw Lewis Structures Class 11 Chemistry Chemical Bonding Youtube
NACL3 Lewis structure. A couple problems with this question: 1. There is no such compound as NaCl3. 2. Sodium is an alkali metal and Cl is a halogen, and the two would always form the ionic ...

Electronegativity Why Does Sodium Chloride Nacl Dissolve In Water But Not Silicon Dioxide Tracingcurves
CaO and NaCl have the same crystal structure and approximately the same ionic radii. If U is the Iattice energy of NaCl, asked Jan 4, 2019 in Chemical Bonding and Molecular Structure by Sahida ( 79.8k points)
What is the electron dot structure for NaCl? Sodium atom will loose one electron to gain noble gas configuration and form sodium cation with +1 charge. Chlorine atom will gain one electron to gain noble gas configuration and form chloride ion with -1 charge. In sodium chloride the one electron from sodium metal gets transferred to chlorine atom.

Write The Electron Dot Structure Of Na And Cl Atoms How Do These Atoms Form A Chemical Bond Name The Type Of Bond So Formed Why Does A Compound So Form Have
(a) Complete the Lewis electron-dot diagram of methyl methanoate in the box below. Show all valence c— electrons. 5. Methanamide, CH3NO, is a liquid at 250C. H (a) The complete Lewis electron-dot diagram for methanamide is shown below. so is (i) In the molecule, angle x is not 1800. Estimate the observed angle. Justify your answer.

Topic Covalent Bonding Lewis Dot Diagrams Do Now Identify Bond Type From Formula Auag Co 2 Li 3 Nna 2 S Mg Csf H2oh2o Nacl So 2 Cu Ch 4 Covalentcovalent Ppt Download
The Lewis Structure for the Salt NaCl, shows two ions which have their (Now) outer shells of electrons filled with a complete octet. In the case of the sodium cation, the filled shell is the outermost of the 'core' electron shells. In the Chloride ion, the outer shell of valence electrons is complete with 8 electrons. ...
Draw orbit structure and electron dot diagram of NaCI, MgCl 2 and CaO. Advertisement Remove all ads.
Diagram of bonding in sodium chloride. A sodium atom gives an electron to a chlorine atom. The result is a sodium ion (2,8)+ and a chloride. In Ionic Bonds valence electrons are completely transferred (not shared). Thus, we write the Lewis structure for NaCl as: NaClLewisDot.

What Would Be The Electron Dot Structure Of Carbon Dioxide Which Has Formula Co Sub 2 Sub Chemistry Q A
NaCl + NH3 + CO2 + H2O ® NaHCO3 + NH4Cl 15)In the space draw a Lewis electron-dot diagram for the reactant containing nitrogen in the equation. 16)Explain, in terms of electronegativity difference, why the bond between hydrogen and oxygen in a water molecule is more polar than the bond between hydrogen and nitrogen in an ammonia molecule.
Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library
The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.
A step-by-step explanation of how to draw the NaCl Lewis Dot Structure (Sodium chloride).For NaCl we have an ionic compound and we need to take that into acc...

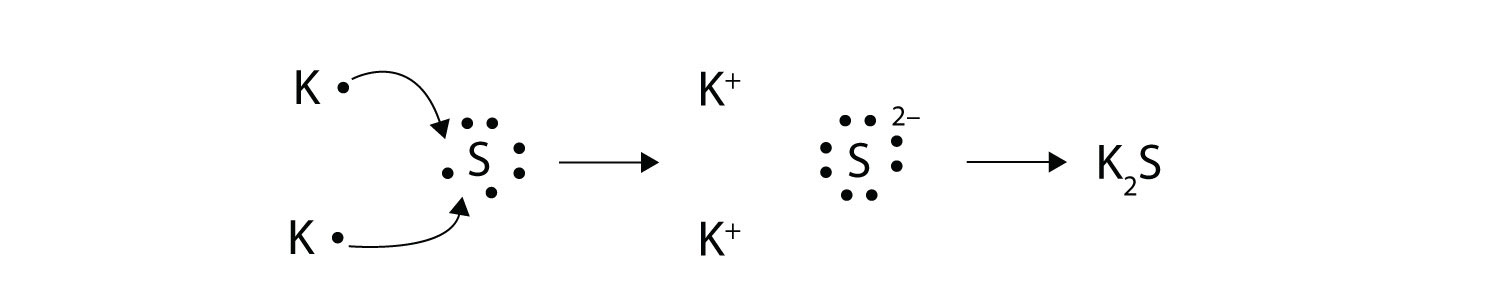
Draw Electron Dot Structures Of Ionic Compounds Nacl Mgcl2 Al2o3 K2o Caf2 Expert Only Science Metals And Non Metals 14968371 Meritnation Com
Are Binary Ionic Compounds Composed Of Metals And Nonmetals Typically From Opposite Sides The Periodic Table Socratic

Draw An Electron Dot Diagram To Show The Formation Of Each Of The Following Compounds Magnesium Chloride H 1 C 6 Mg 12 Cl 17

Give The Formula Of The Stable Compound That Will Be Formed By The Combination Of Na And Cl Using Electron Science Metals And Non Metals 5503251 Meritnation Com






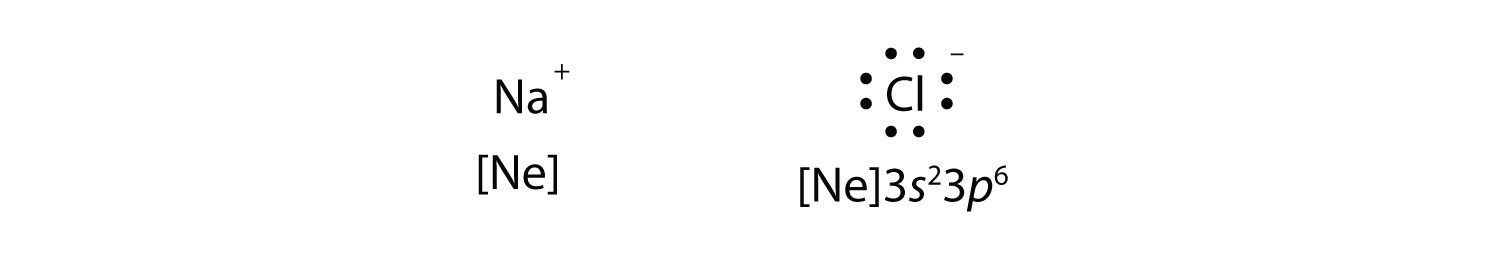












Comments
Post a Comment