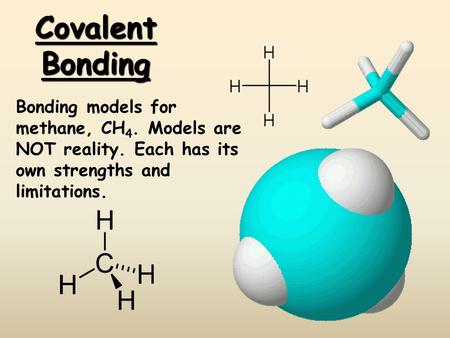
40 ch4 electron dot diagram
How to Draw the Lewis Structure of CH4 (methane). 65K views · 2 years ago ... How to draw dot and cross diagram of methane CH4. Here, the valence electrons of the carbon atom are represented by dot and valence electron of H-atom is represented by *. So, we can clearly see that one electron of carbon and one electron of hydrogen atom combine to form a covalent bond.
Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step.

Ch4 electron dot diagram
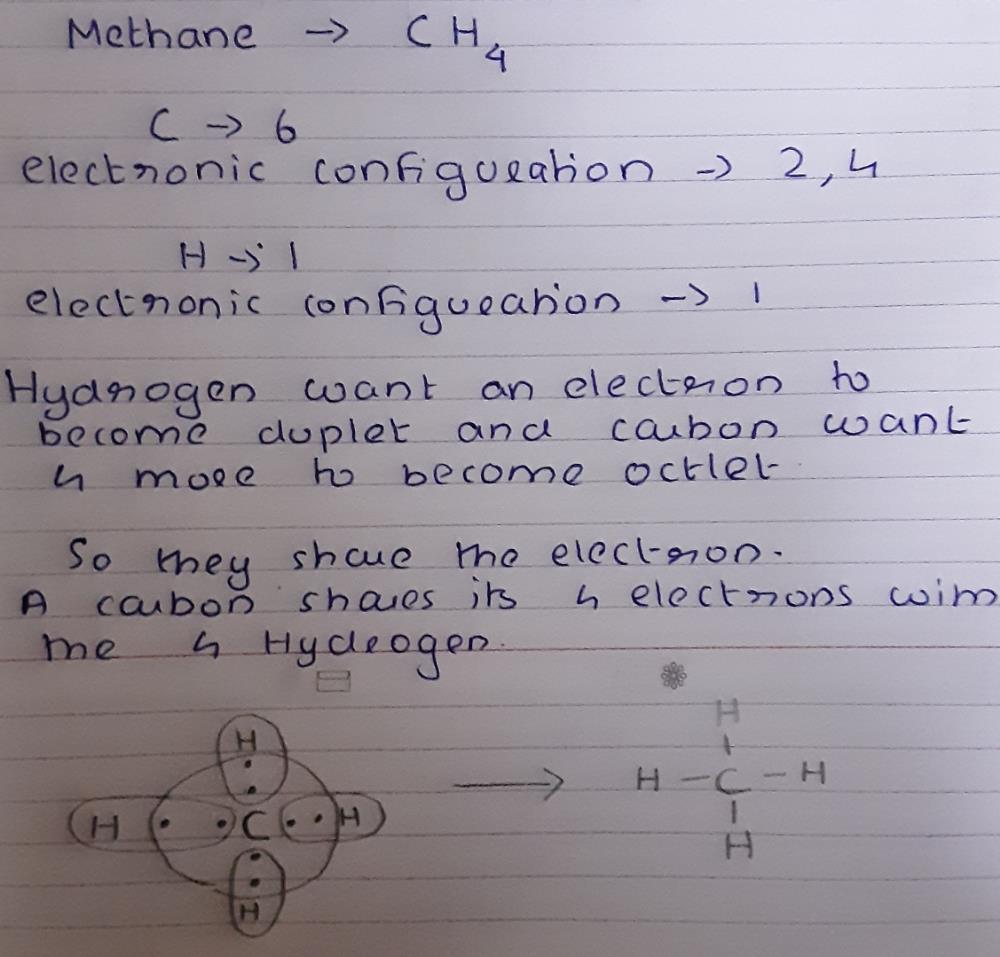
Some possible way to show the structure of CH4 are its electron dot diagram or structural formula. CH4 or methane's molecular formula is given as CH4. The structural formula is a graphical ... In electron dot structure we represent the valence electron of the element. Thus, Carbon has 4 electrons in its electron dot structure and hydrogen has one. They share electrons to form a C − H single bond. 15 Ch4O Lewis Structure. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell. The xe atom is attached to all four oxygen atoms. Worksheet 1 - solutions A - CHEM 2400 Video 1-2…
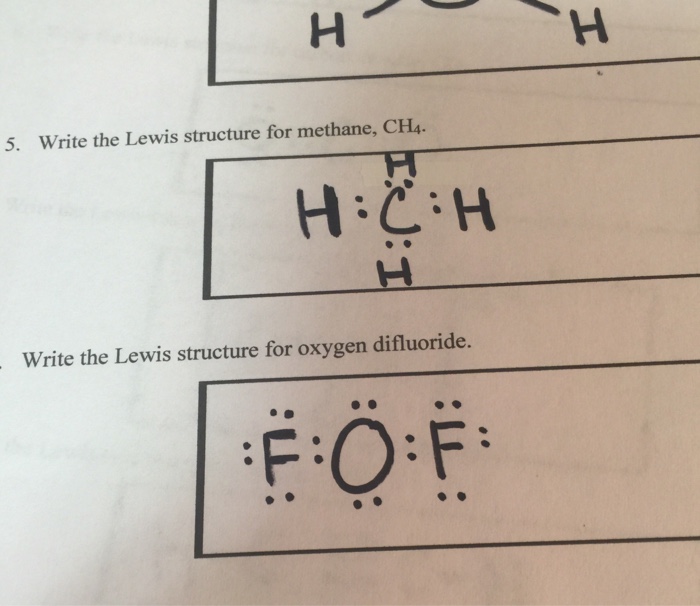
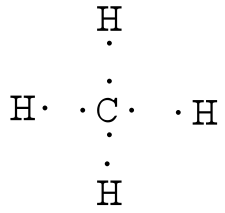
Ch4 electron dot diagram. Thus, Carbon has 4 electrons in its electron dot structure and hydrogen has one. They share electrons to form a C−H single bond. How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use... Answer: Well Carbon only has 4 valence electron, so it can bond at all four point. Hydrogen only has one valence electron and can only share one. Methane's (When it comes to hydrocarbons, "meth" stipulates one carbon, "ane" stipulates a single bond shared with hydrogens) molecular formula is CH4,... Lewis Dot Structures 1. Methane - CH 4 Number of Valence Electrons: 4 from C and 1 each from 4 H = 8 Carbon is more electronegative than hydrogen, but hydrogen can never be the "central" atom, as it can only form 1 bond. Carbon always forms 4 bonds (2 electrons each). 2. Ammonia - NH 3
Draw the Lewis structure for CH 4 using the easy method where you calculate the total valence electrons in the molecule to determine the Lewis structure. The 2s orbitals and one of the 2p orbitals, for suppose, the 2py can hybridize and produce 2 Consult with your instructor, and then complete the rest of the table. Dec 02,2021 - Draw the electron dot diagram of a methane molecule [C=6,H=1]? | EduRev Class 10 Question is disucussed on EduRev Study Group by 589 Class 10 ... Finding CH 4 molecular geometry using VSEPR theory is not very difficult using these three steps. Lewis structure of CH 4. The Lewis structure of the methane CH4 molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Give the Lewis dot structure of eqCH_4 eq. The chemical formula of methane is CH4. It is a gas that exists abundantly in nature. Now, let's know its structure: Lewis dot structure is a representation of ...
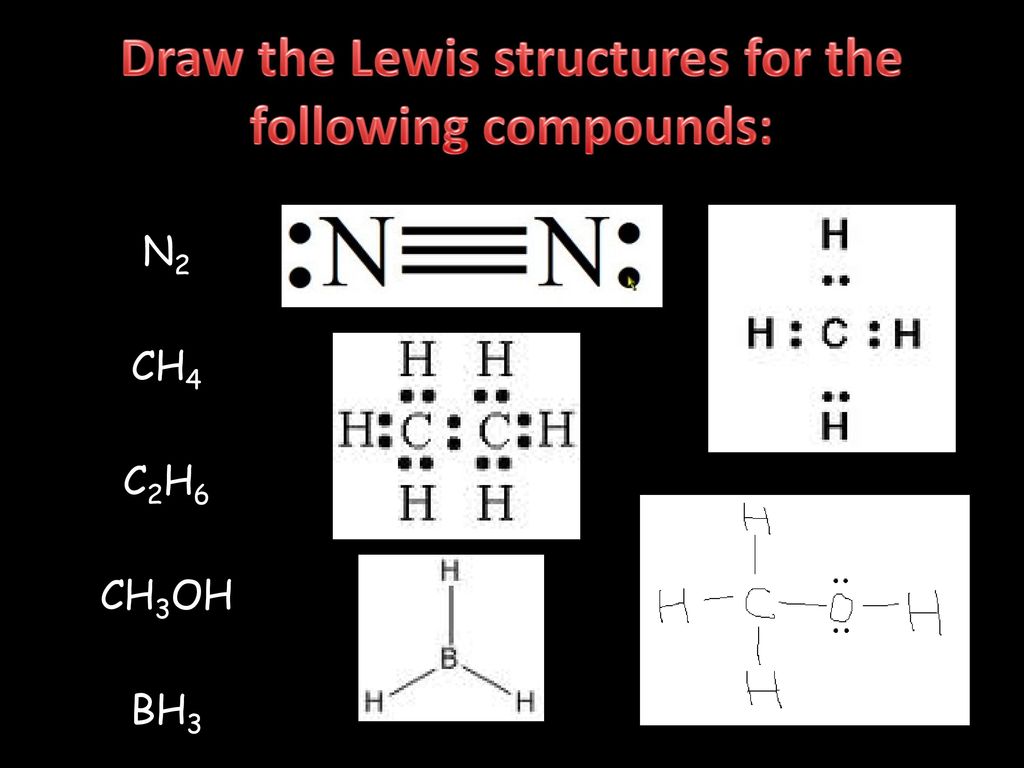
Draw the electron dot diagram of chemical bonds in methane (CH4) and ethane (C2H6). Ch4 Electron Dot Diagram. I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the. Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . Lewis dot structure of CH 4. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C-4 H-1x4=4. Total=8. Put carbon in center and arrange hydrogen atoms on the schematron.orge electrons between carbon and hydrogen atoms. Lewis structures, also known as Lewis dot diagrams ... What is the Lewis dot structure for methane? The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
Lewis Dot Structure for CH4 How to create a Lewis Dot Structure for CH4 # 2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1. How to draw the Lewis structure of methane, CH4 By José @ Periodic table with names diagramweb.net But seriously, you have an electron pair between the C and each of the H's in the ...
The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to two (this is why hydrogen only needs two).The covalent bonds between the C and the H are similar to the ones formed between two Hs ...
The electron dot structures are used to show the bond formation among the elements of a covalent chemical compound. · Similarly, the CH4 is also ...
The electron dot structure of the CH4 molecule is also known as the CH4 Lewis structure. It determines the number of outermost valence electrons as well as the electrons engaged in the CH4 molecule's bond formation. The outermost valence electrons of the CH4 molecule must be understood while considering the Lewis structure of the molecule.
Structure and Bonding. Atomic and Molecular Orbitals. • Atomic Orbitals - s orbitals. ShowMe is an open learning community featuring interactive lessons on a variety of topics. Lewis Structures for CH4. Step-by-step tutorial for drawing the Lewis Structure for CH4. The Lewis Dot Structure for CH4 is shown above.
The lines represent bonds. This is the final Lewis Dot Diagram. The pairs of dots represent shared electrons and you can see that the Carbon ...
CH4 Lewis Structure. Answer: CH4 Lewis structure (Methane electron dot structure) is that type of diagram where we show the total 8 valence electrons of CH4 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ].
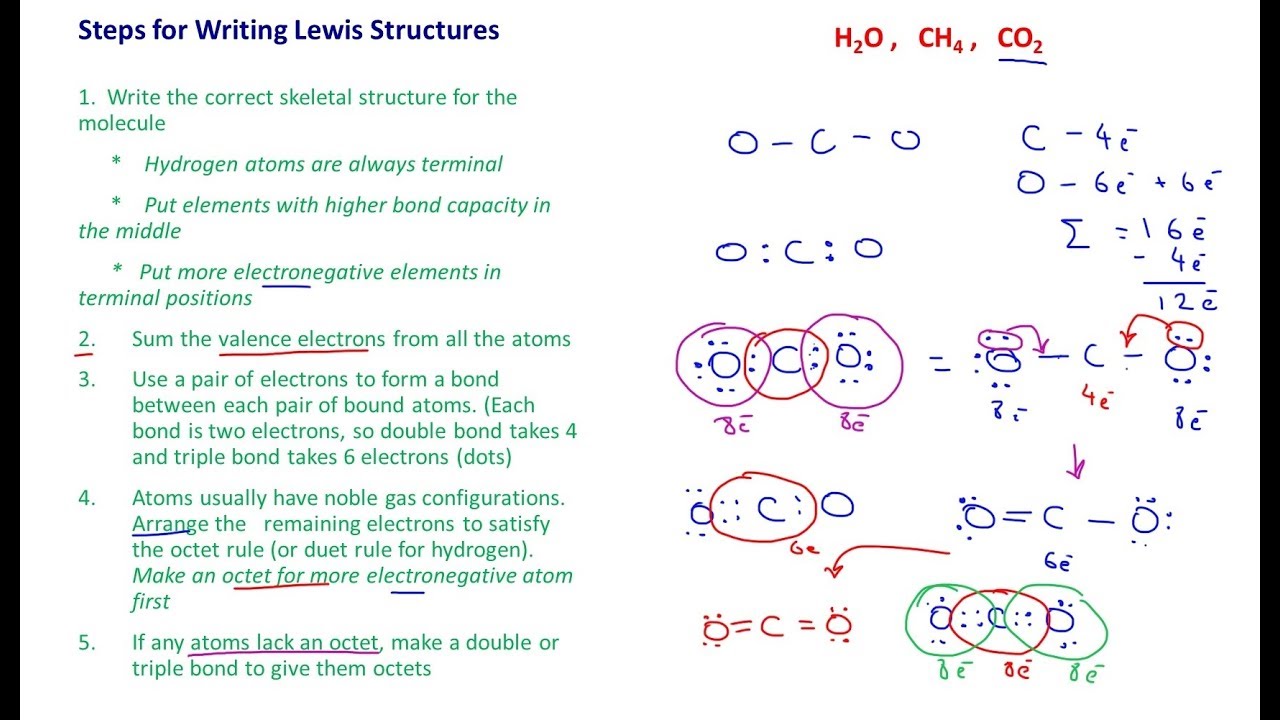
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4.
Methane (CH4) lewis dot structure, molecular geometry, electron geometry, polar or nonpolar, bond angle Home > Chemistry Article > CH4 lewis structure and its molecular geometry Methane is a colorless and odorless gas formed from one atom of carbon and four atoms of hydrogen having the chemical formula CH4.
A step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use the periodic table to find the total number of vale...
CH4 Lewis Structure Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule, which helps us understand the atoms' bond formations. The electrons that participate in bond formation are called the bonding pair of electrons, while those that don't are known as nonbonding pairs of electrons.
Methane (CH4) - Methane is the simplest hydrocarbon with the molecular formula CH4. It is a colourless, flammable gas. Methane is produced naturally from rotting vegetation in marshes.
Answer: I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the middle is the one Carbon, and the four yellow ones are Hydrogens. Now I have drawn the Valence Electrons. These are the blue dots next to the...
Draw The Electron Dot Structure Of Ch4. 34 Electron Dot Diagram For Methane Wiring Diagram Database. 34 Electron Dot Diagram For Iodine Wiring Diagram Database. Electron Dot Diagram For Methane Atkinsjewelry. 3 Ways To Draw Lewis Dot Structures Wikihow.
Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
What is the electron dot structure for CH4? The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
Methane (CH4) ... Diagram. Draw Lewis dot diagram for the following. Methane (CH4) ... Concept: Kossel and Lewis Approach to Chemical Bonding. Report Error
The Lewis dot structure for CH 4 shows the number of valence electrons around each atom. Each dot represents a valence electron. The number of valence electrons is the same in the group and equal to the group number. Reset this page
15 Ch4O Lewis Structure. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell. The xe atom is attached to all four oxygen atoms. Worksheet 1 - solutions A - CHEM 2400 Video 1-2…
In electron dot structure we represent the valence electron of the element. Thus, Carbon has 4 electrons in its electron dot structure and hydrogen has one. They share electrons to form a C − H single bond.
Some possible way to show the structure of CH4 are its electron dot diagram or structural formula. CH4 or methane's molecular formula is given as CH4. The structural formula is a graphical ...
Draw An Electron Dot Diagram To Show The Formation Of Each Of The Following Compounds I Methane Sarthaks Econnect Largest Online Education Community










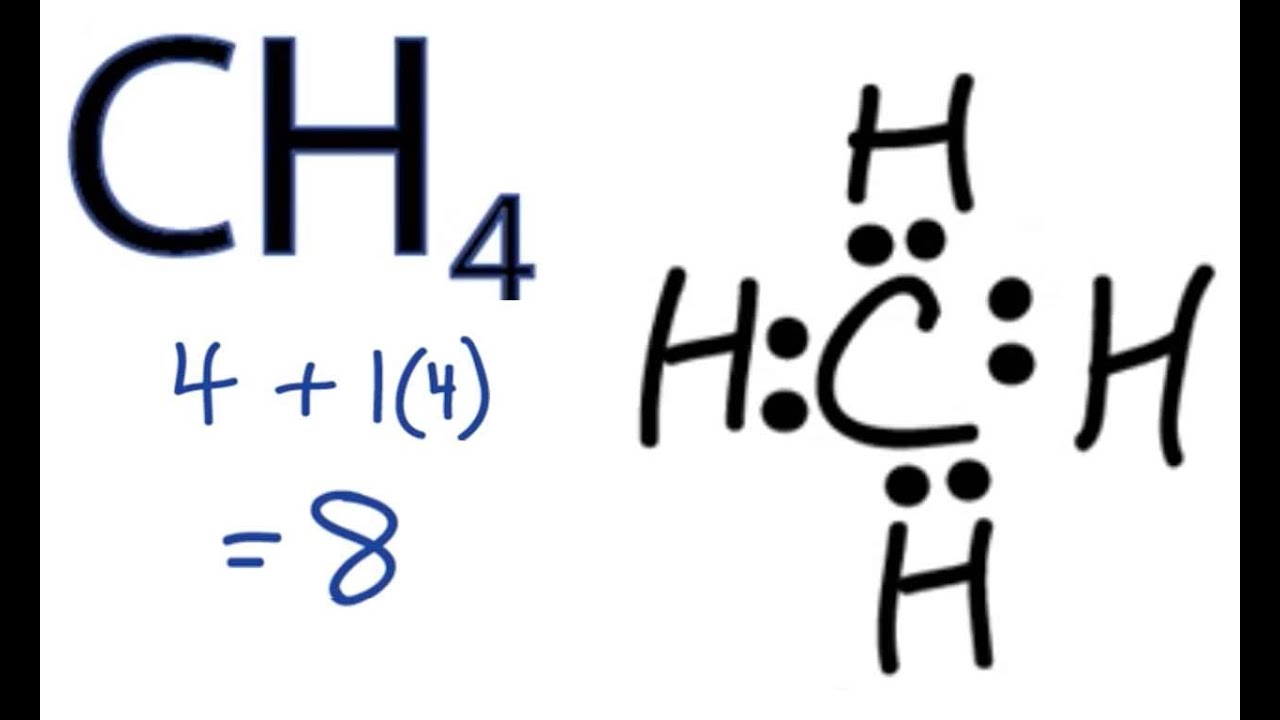









Comments
Post a Comment