41 lewis dot diagram of nitrogen
Lewis Dot Structures: Lewis dot structures are representations used to illustrate the structure of an atom, molecule or ion. It can also be used to predict the properties of these substances. Lewis Structure (electron dot diagram) for ammonia OR Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom.
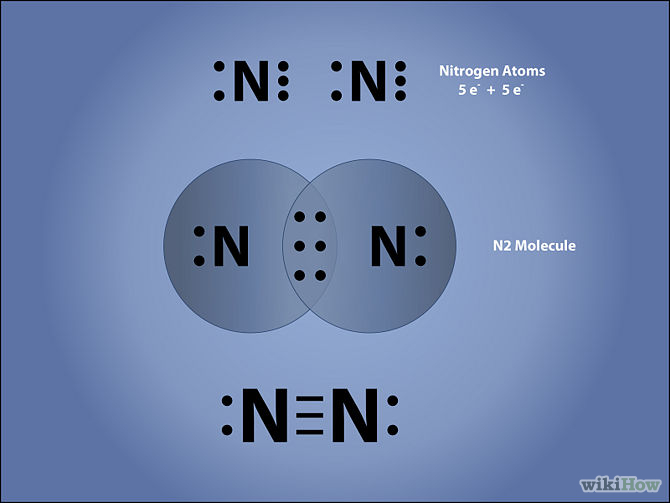
Note: The most important thing about the Lewis dot structure is that only valence electrons take part in chemical bonding. Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2.

Lewis dot diagram of nitrogen
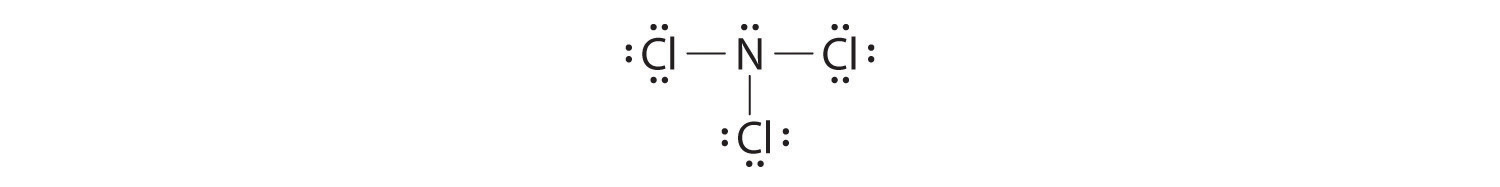
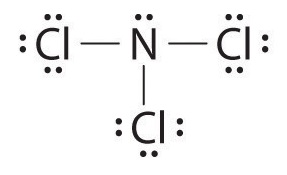
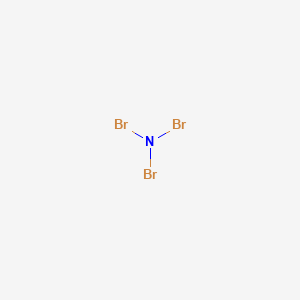
Nitrogen trichloride (NCl3) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Nitrogen trichloride is a very explosive substance that appears like an oily liquid with the chemical formula NCl3. It smells similar to chlorine. It has a dipole moment of 0.6 D that shows it is moderately polar. Nitrate ion lewis dot structure. In brief we need to master 4 steps for making a correct Lewis dot structure. Count total valence electrons in the molecule or ion. Select the central atom and make a skeleton of the molecule or ion. Complete the octet of the most electronegative atom with minimum formal charges. Nov 19, 2018 · The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter N's, so that there would be 6 dots total What is the Lewis dot structure for N2 look like?
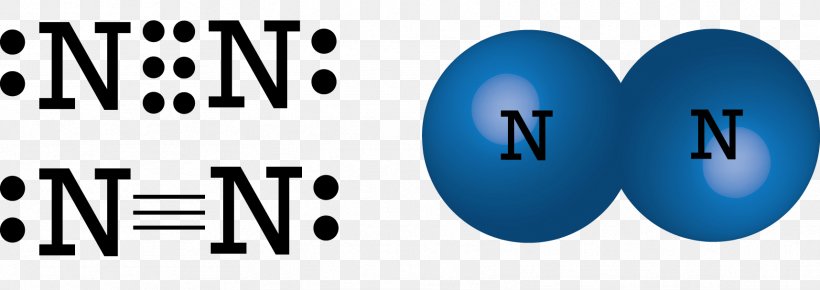
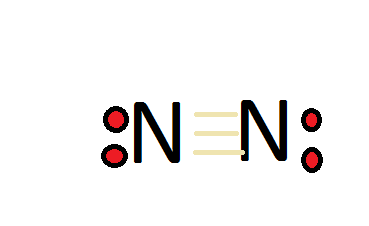
Lewis dot diagram of nitrogen. Drawing the Lewis Structure for N 2 (Dinitogen or Nitrogen Gas). Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N 2).It also is a good example of a molecule with a triple bond. There are 10 valence electrons available for the Lewis structure for N 2.. Video: Drawing the Lewis Structure for N 2 For diatomic nitrogen, the Lewis-dot structure correctly predicts that there will be a triple bond between nitrogen atoms: This triple bond is very strong. The strength of the triple bond makes the N 2 molecule very stable against chemical change, and, in fact, N 2 is considered to be a chemically inert gas .There is a relationship between the ... Transcript: For the HCN Lewis structure we have one valence electron for Hydrogen, we have four for Carbon, and we have five for Nitrogen, for a total of ten valence electrons for the HCN Lewis structure. We'll put the Carbon in the center, because it's less electronegative than the Nitrogen, and Hydrogens always go on the outside of Lewis structures. Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
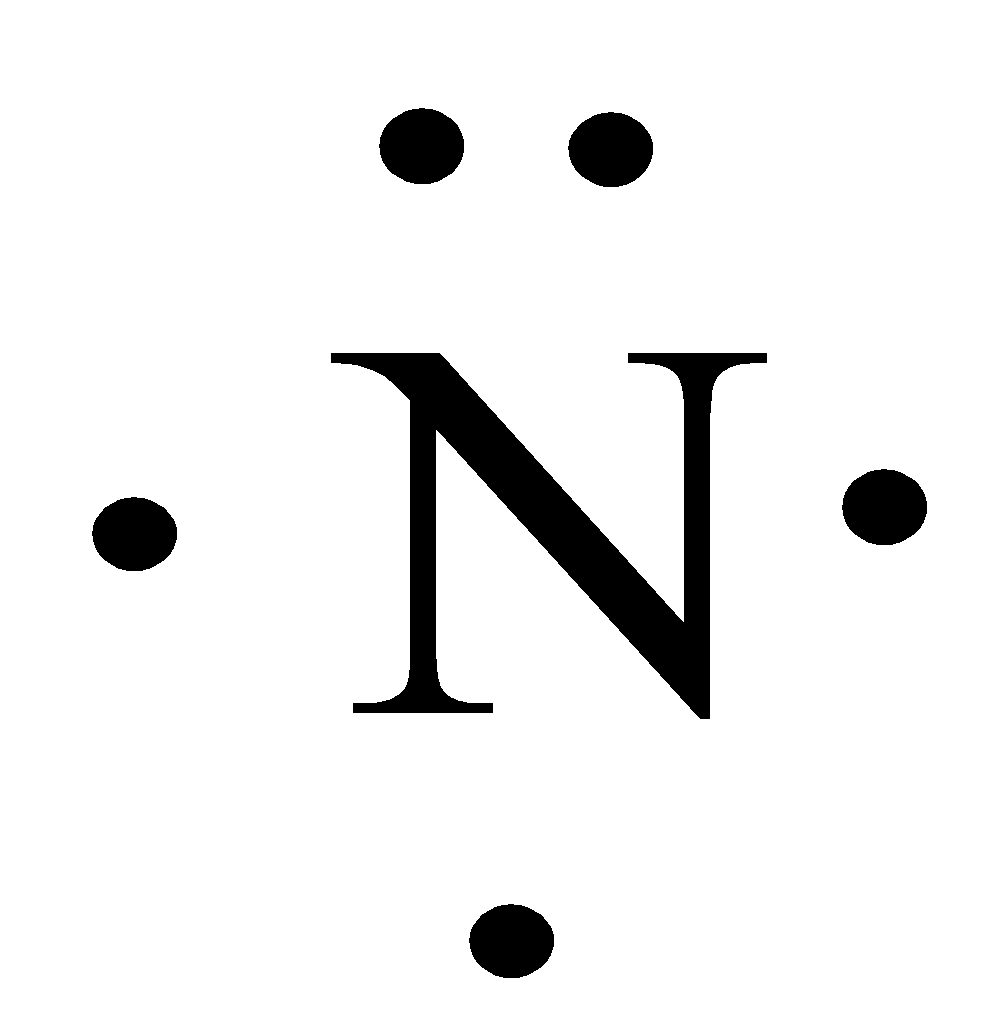
Dec 17, 2009 · The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. N . So, nitrogen is the central atom that has 1 lone pair and 3 bonded pair electrons according to the NF3 lewis dot structure. Hence the formula of NF3 becomes AX 3 N 1 So, according to the VSEPR chart , if the molecule has the formula of AX 3 N 1 , it has a molecular shape of trigonal pyramid and electron geometry of tetrahedral. What is the electron dot diagram for nitrogen? Note: Nitrogen is in Group 5 (sometimes called Group V or Group 15). Since it is in Group 5 it will have 5 valence electrons. When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N). A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
N2 Lewis Structure The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair. N2 Lewis Structure Setup It's easiest to think in terms of […] Lewis Dot Structure of mono-atomic ions Lewis electron dot structure of Nitride ion. Electronic dot structure of Nitride Ion: Electronic configuration for nitrogen =1s22s22p3 In order to complete the octet rule, nitrogen takes three electrons and attain -3 charge.So. Answer (1 of 2): NO3- is the nitrate ion, and the conjugate base of nitric acid.Two of the three oxygens bound to the nitrogen atom have their three outer lone pairs, with a sigma bond to the nitrogen. One of the oxygens is the exception and has only two lone pairs a since it has a double bond co... A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Diatomic Nitrogen).For the N2 structure use the periodic table to find the total number...
Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules. 3. Determine the electron and molecular geometry of the produced molecules. Background: Scientists often create models to represent either a physical or ...
NF 3 lewis structure. In the lewis structure of NF 3, there are three N-F bonds and one lone pair on nitrogen atom which is the center atom.Each fluorine atom has three lone pairs. Steps of drawing lewis structure of NF 3. You have to follow few steps to draw the lewis structure of NF 3.Because nitrogen trifluoride is a simple molecule, these steps are not complex and do not require all steps ...
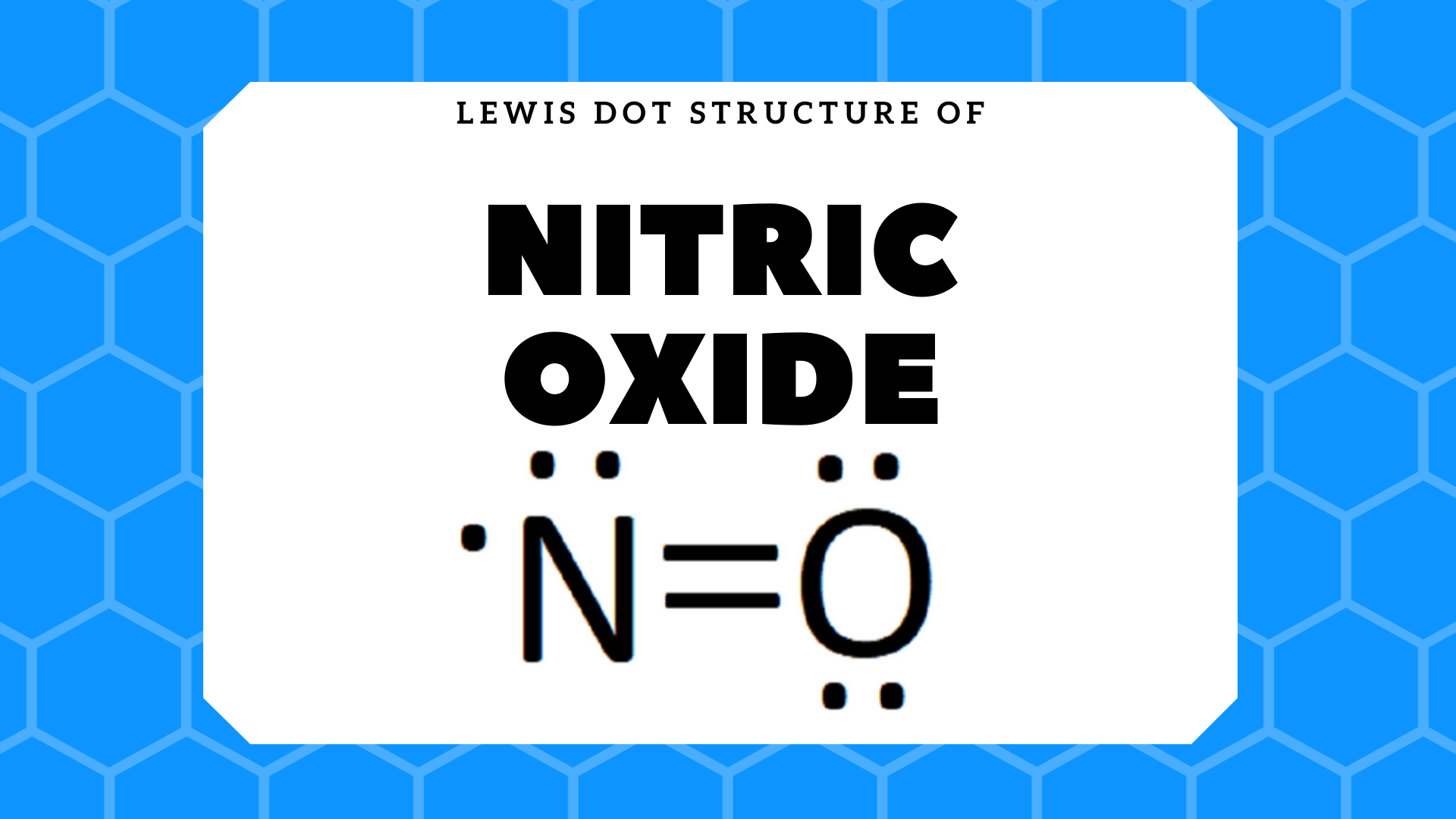
A Lewis structure for NO would look like: Nitric oxide is composed of a single nitrogen atom that is bonded to a nitrogen atom. The double bar between the two chemical symbols (=) means that nitrogen and oxygen share a double bond—2 pairs of electrons. Lastly, there is a single unpaired electron on the nitrogen atom.
Dinitrogen tetroxide is a one of the oxide of nitrogen and are two nitrogen atoms are located at center of the molecule. In Lewis Structure of N2O4, two oxygen atoms have connected to one nitrogen atom. There are charges on atoms in Lewis Structure of N2O4.
NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.
What is the Lewis dot structure for ammonia? In the lewis structure of ammonia (NH3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Can you draw the electron dot structure of […]
A step-by-step explanation of how to draw the Lewis dot structure for N (Nitrogen). I show you where Nitrogen is on the periodic table and how to determine ...

3280 N2 Lewis Structure How To Draw The Lewis Structure For N2 Nitrogen Gas Youtube Nitrogen Gas Lewis
Lewis Dot Diagram for Nitrogen. what is lewis dot diagram of nitrogen gas answers the lewis dot structure of a nitrogen atom would be the capitol letter n with the five valence electrons represented by two dots above it one to the lewis dot structure for nitrogen atom n a step by step explanation of how to draw the lewis dot structure for n nitrogen i show you where nitrogen is on the periodic ...
A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t...
The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen. The electronic configuration of Nitrogen is 1s^2 2s^2 2p^3 The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Nitrogen atom has 5 valence electrons, so its Lewis dot symbol for N is This video shows how to use the periodic ...
what is the Lewis structure of NO nitrogen monoxide, Lewis structures of nitrogen monoxide, Lewis electron dot structures of nitrogen monoxide, electron dot structures of nitrogen monoxide, NO Lewis structures, NO electron dot structures, NO dot structures, pi an d, for the draw, lewis no, dot structure of NO, electron dot lewis structure of NO, resonance structures of NO, ap chemistry lewis ...
This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17.

Hybridization Of Orbitals And Forming Of Bonds In The Nitrogen Dioxide Molecule Chemistry Stack Exchange
Nov 19, 2018 · The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter N's, so that there would be 6 dots total What is the Lewis dot structure for N2 look like?
Nitrate ion lewis dot structure. In brief we need to master 4 steps for making a correct Lewis dot structure. Count total valence electrons in the molecule or ion. Select the central atom and make a skeleton of the molecule or ion. Complete the octet of the most electronegative atom with minimum formal charges.

Nitrogen Molecule N2 Lewis Dot Cross Electronic Diagram Covalent Bonds Ball Stick Space Filling 3d Models Boiling Point Melting Point Doc Brown S Chemistry Revision Notes
Nitrogen trichloride (NCl3) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Nitrogen trichloride is a very explosive substance that appears like an oily liquid with the chemical formula NCl3. It smells similar to chlorine. It has a dipole moment of 0.6 D that shows it is moderately polar.
Lewis Structure Nitrogen Chemical Bond Triple Bond Covalent Bond Png 1716x607px Lewis Structure Atom Atomic Orbital







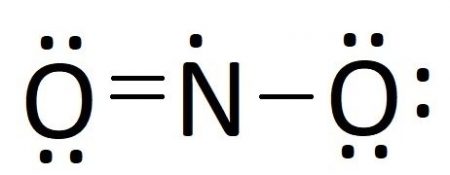

:max_bytes(150000):strip_icc()/NO2_Dot-56a12a2c3df78cf772680359.png)


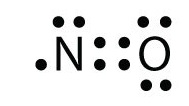






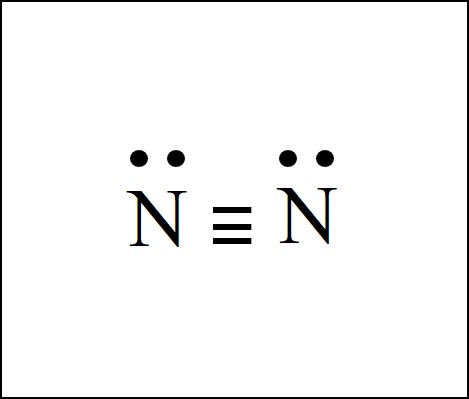
Comments
Post a Comment