39 lewis dot diagram phosphorus
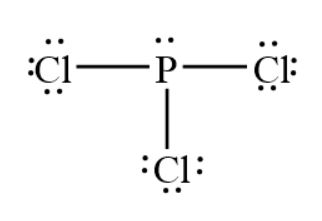
PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and Hybridization. ... 07 Feb Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus tribromide or Pbr3 molecule consists of a phosphorus atom and three atoms of bromine. Phosphorus has an atomic number of 15 and therefore has a valency of 5. In the case of Br, it belongs to the family of halogens and consists of seven valence electrons. Total valence electrons in a single molecule of PBr3 = 5 + 7*3. = 5 + 21.
Draw the Lewis dot diagram for the phosphorus atom. P. Draw the Lewis dot diagram for the CO, 2 ion: 9 Give the electron conjuration (. for the Mog"lton. Which has the larger radius. F of F Which has the larger radius (car Ca+2? Arrange in order of...

Lewis dot diagram phosphorus
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. Pcl3 Lewis Dot Structure. Here are a number of highest rated Pcl3 Lewis Dot Structure pictures upon internet. We identified it from obedient source. Its submitted by paperwork in the best field. We bow to this kind of Pcl3 Lewis Dot Structure graphic could possibly be the most trending subject as soon as we portion it in google lead or facebook. Lewis structure of Phosphorus Trifluoride PF3 The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. Phosphorus has 5 valence electrons while the 3 fluorine atoms have 21 valence electrons 7 for.
Lewis dot diagram phosphorus. how to draw the lewis dot structure for h3po3 phosphorous. Phosphorus Dot Structure. Here are a number of highest rated Phosphorus Dot Structure pictures upon internet. We identified it from obedient source. Its submitted by giving out in the best field. We resign yourself to this kind of Phosphorus Dot Structure graphic could possibly be the ... There are 26 valence electrons for Phosphorus Tribromide. PBr3 Lewis Structure Lewis dot structures or Lewis structures are the diagrams that help to understand the bonding of atoms along with the lone pairs present in the molecule. The valence electrons of atoms form bonds, and these bonds are represented by showing straight lines. PF3 is a nucleophile where it donates a pair of electrons during a chemical reaction. Lewis diagram is a pictorial presentation of the number of valence electrons present in an atom, which readily reacts with the valence electrons of another atom to form a bond. The diagram is drawn with the help of eight dots around the atom, mostly in pairs. The Lewis electron dot diagram is a representation of valence electrons of an atom that uses dots around the symbol of element. The number of dots equals the number of valence electrons in an atom. These dots are arranged to right and left and above and below the symbol, with NO more than two dots on side.
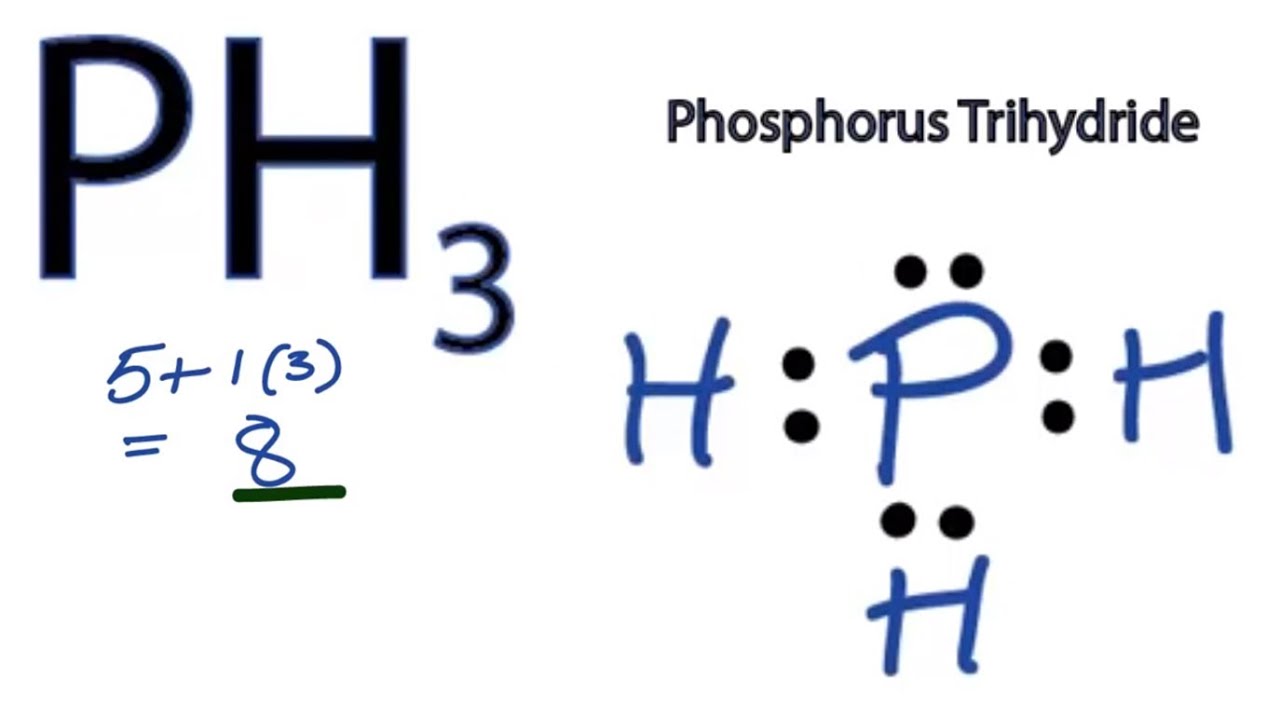
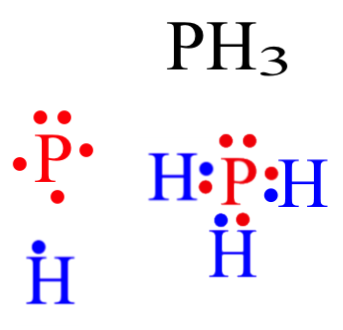
Lewis structure, also called electron-dot structure, is a structural formula in which electrons are represented by dots; two dots between two atoms represent a covalent bond. In fact, Lewis structures are very important for predicting geometry, polarity and reactivity of (in)organic compounds. The the Lewis dot and cross electronic diagram used to of SiH4 bond angles H-N-H VSEPR molecule shape of NH4+ bond angles VSEPR BeCl2 (X = Be, Q = F, Br, Cl or I). BH3. electrons in the valence shell of the central atom of the molecule or Be and B don't need 8 valence electrons. Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen. To answer your question, you would draw the resonance structure of each Lewis Dot Structure and then apply Formal Charge calculation to see which structure is more correct. Any element that reaches the 3d shell will have an expanded octet. So in a sense, even Phosphorus can go beyond 8 electrons.
SF4 Lewis Structure Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. This Lewis structure has eight electrons - one lone pair on phosphorus 2 and three bonds 6. The number of valence electrons is 4 6 10 electrons or 5 pairs. Carbon C EN 25 Fluorine F EN 40 C is the central atom. What is the Lewis dot structure for pcl3? PCl3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl3) has 3 chlorine atoms as well as one phosphorus atoms. In PCl3 lewis structure, each chlorine atom is joint with facility phosphorus atom with a solitary bond. Likewise, there is an only set on phosphorus atom. This leads to the following Lewis dot structures. Question 10. Draw the Lewis dot structure of CO 3 ion. Answer: Total no. of valence electrons of CO 3 = 4 + 3 × 6 = 22 total no. of valence electrons on CO 3 = 22 + 2 = 24 The skeletal structure of CO 3 is Putting one shared pair of electrons between C and O and completing the Octets of oxygen ... Electron Dot Structures Teachers Answer Key. Worksheet Lewis Dot Structure Worksheet Answers electron dot diagrams and lewis structures worksheet answers 9th higher. Electron point diagram and worksheet structure lewis. Worksheet Electron Dot Diagrams and Lewis Structures Responses Lobo Magnesium iodine boro sulphur carbon phosphorus ii.
Lewis structure of Phosphorus Trifluoride PF3 The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. Phosphorus has 5 valence electrons while the 3 fluorine atoms have 21 valence electrons 7 for.
Pcl3 Lewis Dot Structure. Here are a number of highest rated Pcl3 Lewis Dot Structure pictures upon internet. We identified it from obedient source. Its submitted by paperwork in the best field. We bow to this kind of Pcl3 Lewis Dot Structure graphic could possibly be the most trending subject as soon as we portion it in google lead or facebook.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.





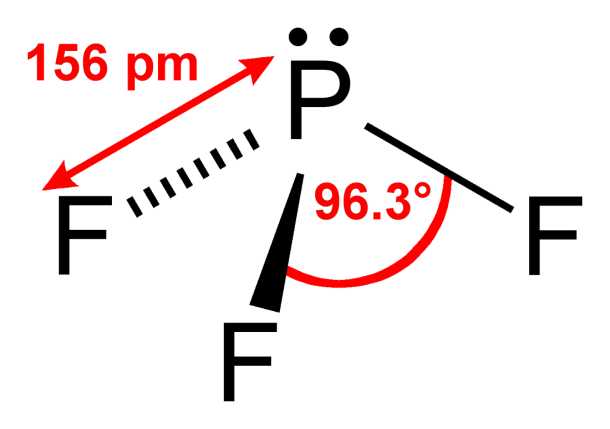


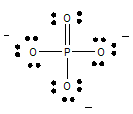












Comments
Post a Comment