40 arsenic dot diagram
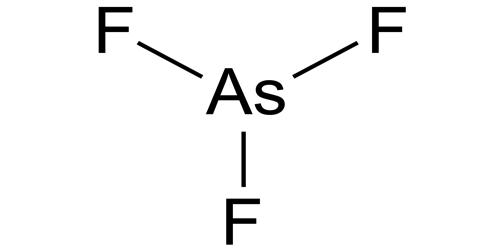
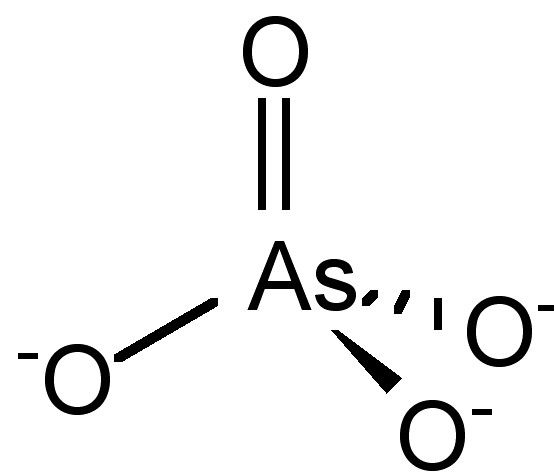
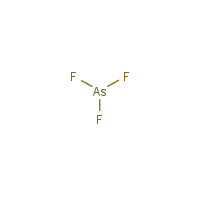
The Lewis structure for AsCl 3 is similar to AsF 3 structure. Since they are in the same Group on the periodic table they each have the same number of electrons their structures are similar. The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom. For the AsCl 3 Lewis structure there are a total of ... The complete Lewis dot structure is shown in the above figure. Molecular Geometry of AsF5. Arsenic pentafluoride has a trigonal bipyramidal molecular geometry which is considered as a symmetrical geometry. And this is the main reason why AsF5 is a nonpolar molecule although it has five polar bonds in the form of As-F.
Phosphorus pentoxide | P2O5 or O5P2 | CID 6326975 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological ...
Arsenic dot diagram
DRAWING LEWIS DOT DIAGRAMS 1) Given a Bohr model, count the 2 Mg DRAWING ELECTRON DOT DIAGRAMS Draw the Dot Diagram for arsenic (As). Comprehensive information for the element Arsenic - As is provided by this page including scores of Atomic Structure of Arsenic Electron Dot Model. The left diagram shows a Lewis dot structure of sodium with. 1. Write the electron dot structure (Lewis Dot Structure) for covalent compounds or ions. 2. Use electronegativity to determine the polarity of a bond or molecule. 3. Given the formula of a covalent compound, write its correct name; given the name of … 1 answerThere should be 5 dots on the electron dot structure of arsenic- Arsenic is in column VA in the periodic table so it will have 5 valence electrons. T...
Arsenic dot diagram. The Lewis structure for AsH 3 is similar to AsF 3 structure. The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom. Remember that Hydrogen (H) atoms always go on the outside of a Lewis structure. For the AsH 3 Lewis structure there are a total of 8 valence electrons available. A solar cell, or photovoltaic cell, is an electrical device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon. It is a form of photoelectric cell, defined as a device whose electrical characteristics, such as current, voltage, or resistance, vary when exposed to light. Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal.Arsenic is a metalloid.It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry.. The primary use of arsenic is in alloys of lead (for example, in car ... A step-by-step explanation of how to draw the AsBr3 Lewis Dot Structure.For the AsBr3 structure use the periodic table to find the total number of valence el...
27 Jan 2021 — Arsenic Valence Electrons | Arsenic Valency (As) with Dot Diagram ... Get to study the Arsenic valence electrons here to get better in chemistry. Arsenic triiodide | AsI3 | CID 24575 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety ... Electron Dot Diagram for Halogens. 7. ... The nonmetals of the nitrogen family (nitrogen, phosphorous, arsenic) form ions with a charge of. 3-The nonmetals of the oxygen family (oxygen, sulfur, selenium) form ions with a charge of. 2-All of the elements of the alkali metals group form ions with a charge of. 1+ Draw a dot and cross diagram for a molecule of carbon dioxide. Show outer electrons only. (2) ... Draw a labelled diagram of the apparatus used to filter the mixture and collect the sodium chloride solution. (2) ... 75 As arsenic 33 79 Se selenium 34 80 Br bromine 35 84 Kr kr yp ton 36 85 Rb rubi di um 37 88 Sr strontium 38 89 Y yt triu
How do you draw electron dot diagrams? How to Draw a Lewis Dot Structure. Determine the total number of valence electrons to be depicted in the Lewis diagram. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Determine how many electrons must be added to central element. Zintl and E. Huseman, "Crystal Structure and Lattice Parameters of Binary Magnesium Compounds," Z. Phys. Chem., B 21, 138-155 (1933) in German. R. Juza and R. Kroebel, "On a High-Temperature Modification of Magnesium-Arsenide and a Ternary Phase Mg 2 MnAs 2 of Similar Structure," Z. Anorg. Arsenic pentafluoride (AsF5) lewis dot structure, molecular geometry, hybridization, polar or nonpolar Home > Chemistry Article > AsF5 lewis structure and its molecular geometry Arsenic pentafluoride is a chemical compound made up of arsenic and fluorine. Arsenic pentafluoride forms halide complexes and is a powerful acceptor as shown by the reaction with sulfur tetrafluoride forming an ionic complex. AsF 5 + SF 4 → SF 3 + + AsF 6 − Safety. Arsenic pentafluoride is an extremely dangerous toxin, mainly poisoning liver cells. It has a smell that is similar to vinyl chloride gas. [citation needed]
Atomic Number - Protons, Electrons and Neutrons in Arsenic. Arsenic is a chemical element with atomic number 33 which means there are 33 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
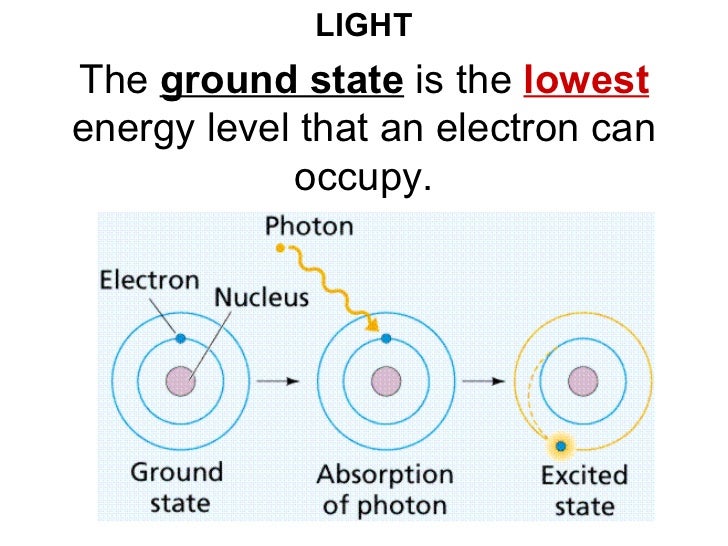
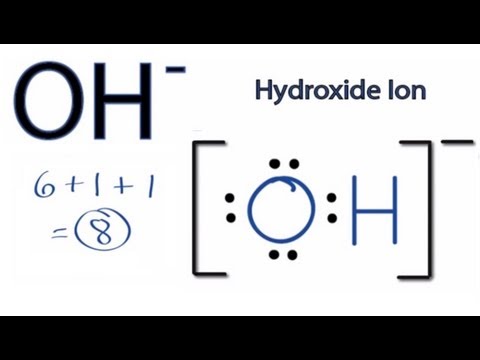
Arsenic is isoelectronic with nitrogen (they are both Group V elements), so there are 5 valence electrons.A handy way to illustrate these valence electrons is to use Lewis diagrams, also called electron dot diagrams. These diagrams show the symbol of the element with as many dots around it as there are electrons in the outermost energy level.
A schematic diagram of the area surrounding the Vredefort Dome, where a massive meteor created an impact crater 300 km in diameter 2020 million years ago. The red dot represents the point of impact. The outer circle has a radius of 150 km, …
(iii) Complete the dot-and-cross diagram to show the electron arrangement in a molecule of NF 3. Use dots for nitrogen electrons and crosses for fluorine electrons. Show outer electrons only. F F N F [3] (c) Lithium nitride melts at 813 °C. Nitrogen trifluoride melts at –206°C.
An electron-dot diagram is a graphical representation of the valence electrons of a certain element. The chemical symbol of an element placed in the middle and the valence electrons are represented by dots/5 (7). Arsenic (As) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
10-01-2022 · We employ turbostratic stacked chemical vapor deposition (CVD) graphene for a mid-wavelength infrared (MWIR) photodetector using the photogating effect. Turbostratic stacked CVD graphene was fabricated by multiple transfer processes. Graphene field effect transistor-based MWIR photodetectors were developed using an InSb substrate.
Answers. Strontium (Sr) and Gallium (Ga) are the elements which have fewer than four dots in the electron dot diagrams. Explanation: Electron dot diagrams are the diagrams which represent the valence electrons in an element. The electrons are represented by the dots in these diagrams. Valence electrons in Arsenic (As) = 5.
Arsenic Bromide Lewis Dot Structure (Marco M, Eric S) by LakeView Chemistry - February 15, 2013.
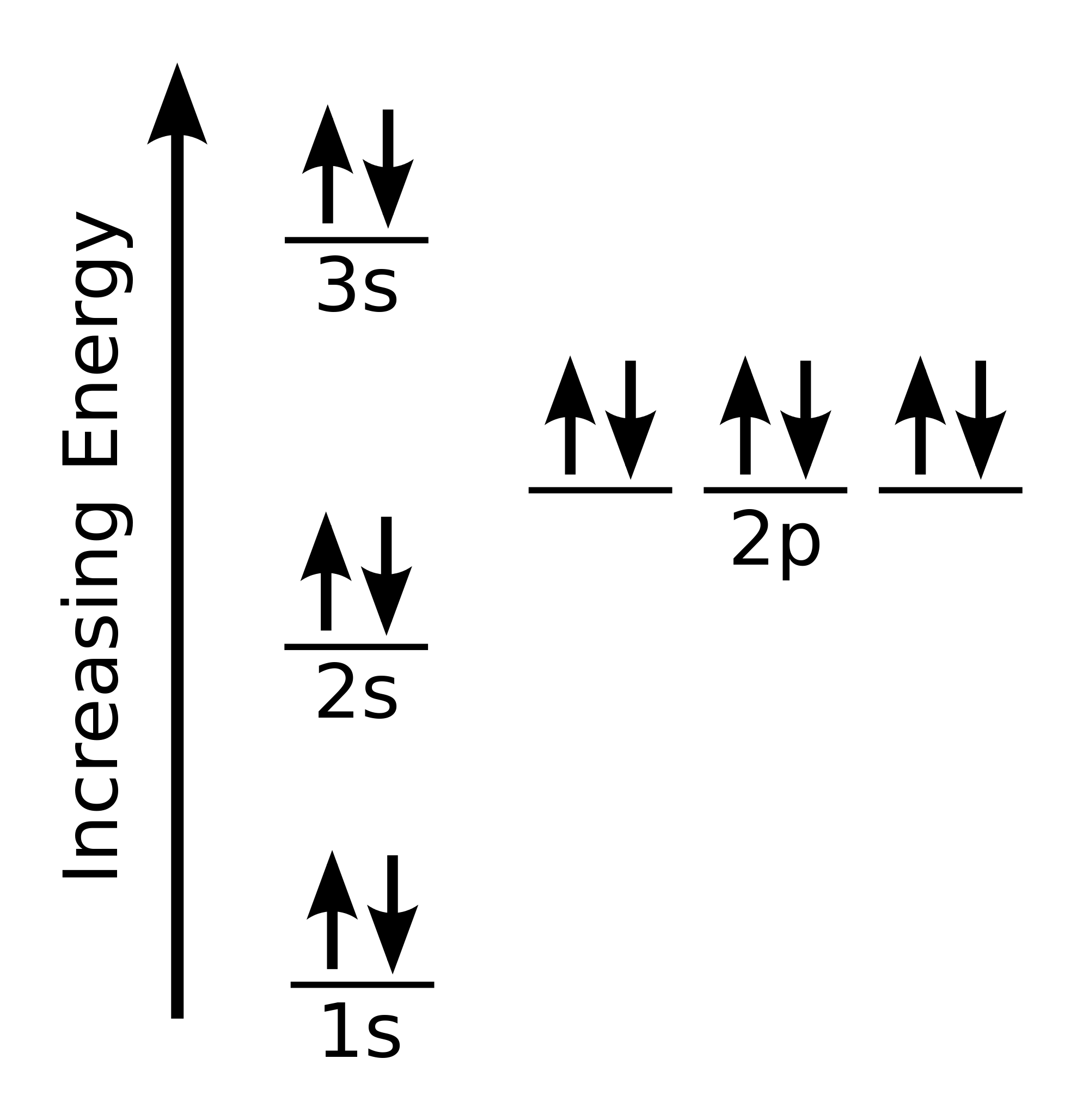
After the 3d sublevel is filled, the remaining three electrons will be in orbitals in the 4p sublevel. Thus, the electron configuration for arsenic is [Ar]4s 2 3d 10 4p 3. Before writing the electron - dot structure for arsenic, note that arsenic's ten 3d electrons are not in the highest principal energy level.
A step-by-step explanation of how to draw the Arsenic (As) Lewis Dot Structure.For the ArsenicLewis structure use the periodic table to find the total number...
Why is this (point to Lewis dot structure of oxygen) an example of a Lewis dot structure? How many electrons or dots should be placed around the symbol of nitrogen in a Lewis dot structure? How do you know? ... Arsenic 6. Iodine. Chemistry 2e (2Q) Draw Lewis dot structures.
4 Jul 2020 — There should be 5 dots on the electron dot structure of arsenic- Arsenic is in column VA in the periodic table so it will have 5 valence ...
Annual world wide production is around 47,000 tons (As2O3). Uses of Arsenic: Used as a deadly poison, in shotgun pellets, metal for mirrors, glass, lasers, light-emitting diodes (LED) and in semiconductors. Additional Notes: Arsenic is a carcinogen, associated with lung cancer when inhaled. Contact with skin can result in skin cancer.
In the AsCl3 Lewis structure diagram, we always begin by introducing valence electrons from the central Arsenic atom (in step1). As a result, wrap around the central Arsenic atom's bond pair valence electrons first (see figure for step1). The Arsenic atom in the molecule gets only 8 electrons around its molecular structure.
Chloroform is a colorless, volatile, liquid derivative of trichloromethane with an ether-like odor. Formerly used as an inhaled anesthetic during surgery, the primary use of chloroform today is in industry, where it is used as a solvent and in the production of the refrigerant freon.
What is the Lewis dot structure for arsenic? The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom. Remember that Hydrogen (H) atoms always go on the outside of a Lewis structure. For the AsH3 Lewis structure there are a total of 8 valence electrons available.
electron dot structures for the atoms listed. So fastly we've got carbon arsenic, bologna and potassium Amberin so we can go ahead and start with carbon.
Sodium's electron-dot structure is because only the 3s electron is a valence electron. The other electrons are in inner energy levels. Example Problem: Writing Electron-Dot Structures. Write an electron-dot structure for a neutral atom of arsenic, an element used in semiconductor materials because of its electron configuration.
The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in its outermost shell. What elements have 5 electrons in their electron dot...
What is the Lewis dot structure for arsenic? Chemical Bonding: AsH3 Lewis Structure The Arsenic atom goes in the center of the Lewis structure since it is the least electronegative atom. Remember that Hydrogen (H) atoms always go on the outside of a Lewis structure. For the AsH3 Lewis structure there are a total of 8 valence electrons available.
Arsenic is very toxic. It is an acute poison, a contact poison, a chronic cumulative poison, and a carcinogen. There is no part of arsenic that is not poisonous. This sample of treated lumber would make you very sick if you ate it. A treated lumber deck has enough arsenic to kill at least a hundred people, including you. Do not use acidic deck ...
Electron Dot Diagram Arsenic Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in These diagrams are used as a. Comprehensive information for the element Arsenic - As is provided by this page including scores of Atomic Structure of Arsenic Electron Dot Model.
25-06-2021 · Hydrogen electron configuration is 1s 1.Hydrogen is a s-block element. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles.. The first element of the periodic table is hydrogen and its position at the beginning …
In the AsH3 Lewis structure diagram, the Arsenic atom can be the center atom of the molecule. As a result, central Arsenic in the AsH3 Lewis structure, with all three hydrogens arranged in the tetrahedral geometry. Add valence electrons around the hydrogen atom, as given in the figure.
30 Apr 2016 · 1 answerArsenic is isoelectronic with nitrogen (they are both Group V elements), so there are 5 valence electrons. Look at the position of arsenic ...
Arsenic is a nonessential trace element that is widely distributed in nature. Arsenic was used in medicinal agents in the 19th and early 20th centuries, but has been replaced by safer and more effective agents and has not been in use for over 50 years. Nevertheless, arsenic is found widely in nature and accidental or intentional acute or chronic exposures to moderate or high levels of arsenic ...
(ii) On the diagram, use one arrow to show where water enters apparatus A. [1] 10 ... Complete the dot-and-cross diagram to show the electron arrangement of a molecule of ethanal. ... N nitrogen 14 15 P phosphorus 31 33 As arsenic 75 51 Sb antimony 122 83 Bi bismuth 209 8
1 answerThere should be 5 dots on the electron dot structure of arsenic- Arsenic is in column VA in the periodic table so it will have 5 valence electrons. T...
1. Write the electron dot structure (Lewis Dot Structure) for covalent compounds or ions. 2. Use electronegativity to determine the polarity of a bond or molecule. 3. Given the formula of a covalent compound, write its correct name; given the name of …
DRAWING LEWIS DOT DIAGRAMS 1) Given a Bohr model, count the 2 Mg DRAWING ELECTRON DOT DIAGRAMS Draw the Dot Diagram for arsenic (As). Comprehensive information for the element Arsenic - As is provided by this page including scores of Atomic Structure of Arsenic Electron Dot Model. The left diagram shows a Lewis dot structure of sodium with.













Comments
Post a Comment