42 co dot diagram
Aug 02, 2021 · A step-by-step explanation of how to draw the Lewis Dot Diagram for CO. Use the periodic table of elements to find the total number of valence electrons for the CO molecule. Once we know how many valence electrons does carbon monoxide have we can distribute them around the central atom with the goal of filling the outer shells of each atom. Dot and cross diagrams for covalent bonding **HIGHER TIER: Any reference to H2O, NH3, CH4, O2, N2 or CO2 bonding is higher tier.** We can use dot and cross diagrams to show how a pair of electrons...
In the CO Lewis structure there aren't enough valence electrons available for each atom to obtain an octet without sharing more than one pair. Therefore CO has a triple bond between the carbon and oxygen atom. For the CO Lewis structure there are a total of 10 valence electrons available.
Co dot diagram
CO2 or carbon dioxide is considered as acid or can even be called Lewis Acid. The resonance structure accepts lone pairs of electrons, but the three lone pairs of electrons are in the oxygen molecule. There are no lone pairs of electrons in CO 2. Although, when dissolved in water, CO 2 takes the form of carbonic acid using this formula. Loop fragment is used to represent a repetitive sequence. Place the words ‘loop’ in the name box and the guard condition near the top left corner of the frame. Sequence Diagram: It is used to surround the whole sequence diagram. Example of a Sequence Diagram. An example of a high-level sequence diagram for online bookshop is given below. The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. Answer : Lewis-dot structure : It represent the number of valance electrons present in ...
Co dot diagram. Jan 17, 2022 · CO Lewis Structure, Geometry, and Hybridization. Carbon monoxide (CO) is a tasteless and odorless flammable gas that is quite toxic in nature to the fauna. It is so because, carbon monoxide uses hemoglobin, an oxygen carrier, to reach throughout the body when in a concentration of more than 35ppm. The carbon monoxide is produced from the ... The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. YouTube. Wayne Breslyn. 407K subscribers. 1. Is Carbon monoxide acidic or basic? Carbon monoxide is a neutral gas since it does not show basic and acidic properties when it reacts with water. 2. Explain CO dot structure in simple words. The overall carbon to oxygen atom ratio in a CO dot structure is 1:1. By sharing three valence electrons, carbon forms three tipple bonds with oxygen. Carbon monoxide (CO) - Carbon monoxide is a colorless gas represented as CO. Visit BYJU’S to understand the properties, structure and uses of CO (Carbon monoxide) explained by India’s best teachers.
Electron dot structures or Lewis dot formula - It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. Electron dot structures of carbon dioxide. The carbon is the central atom of this molecule. Oxygen atom contains 6 valence electrons which form 2 lone pairs. The Lewis Structure (Lewis Dot Diagram) for CO.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill oute... SLD. The primary function of the SLD (Straight Line Diagram) application is to display selected information along a segment of a highway or corridor. The user chooses a segment by specifying a route and milepost interval, then selects the desired roadway information to be symbolized in the diagram. The information is organized into groups based ... blogdc.drupy.co. Dot Sequence Diagram. 1/15/2022 / Comments off. SequenceDiagram.org is an online tool / software for creating UML sequence diagrams. Founded in 2014 with the purpose to improve the efficiency when creating and working with sequence diagrams by combining text notation scripting and drawing by clicking and dragging in the same ...
CO2 Lewis Properties. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a gas. CO 2 has a rather low boiling point of around -80 ℃ or -100 ℉. It can be liquified and even frozen solid with special machinery to produce "dry ice.". A Lewis Dot Structure is drawn by a series of dots, lines, and atomic symbols and provides a structure for the way that the atom or molecule is arranged. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures A step-by-step explanation of how to draw the CO(Carbon Monoxide) Lewis Dot Diagram.For the CO structure use the periodic table to find the total number of v... Carbon monoxide (CO) is a diatomic molecule and contains carbon and oxygen atoms. Lewis structure of CO molecule contains a triple bond. Both Carbon and Oxygen atoms have one lone pair in their valence shells. CO lewis structure. In the lewis structure of carbon monoxide, both atoms have eight electrons in their valence shells.
The Transportation Commission of Colorado and Colorado Department of Transportation are proposing a new standard to reduce greenhouse gas emissions from the transportation sector, improve air quality and reduce smog, and provide more travel options. CDOT, on behalf of the Commission, will hold eight public hearings across the state to provide ...
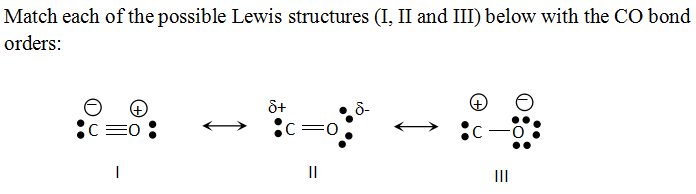
Let us now write the electron dot structure also called as Lewis dot structure for $CO$molecule. - Lewis dot structure or electron dot structure or sometimes also called as Lewis electron dot structure is the diagram which shows the bonding between the atoms of a molecule and the lone pair of electrons that may exist in the molecule.
CO2 lewis structure contains two oxygen atoms and one carbon atom, connected with the double bond whereas carbon is the central atom, and no lone pair is present on it. But each oxygen in the CO2 lewis dot structure has two lone pairs. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule.
The Lewis Dot Structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule. The reason for drawing/creating a Lewis dot structure is that it helps one predict the kinds of bonds, as well as a number of bonds, that can be formed around an atom. Lewis structures can be utilized to make ...
The resulting Lewis electron dot structure displays a triple bond connecting a carbon and an oxygen atom, each holding a lone pair of electrons. Solved Examples. Problem-1: In terms of electron dot formulas, define the electron structure of the carbonate ion CO 3 2-. Solution: One potential electron dot formula for the carbonate ion is
The formula is H 2 O so the dot and cross diagram includes two H atoms and one O atom. H has 1 outer electron. O has 6 outer electrons. The H circles must each overlap the O circle. Question Draw ...
CO Lewis structure, Hybridization, and Molecular Geometry (Carbon Monoxide) Carbon Monoxide is a colorless and odorless gas. This gas is less dense than the air and flammable. People know about this gas, as it can also cause poisoning. Carbon Monoxide is a toxic gas that binds with hemoglobin, which interferes with its binding with Oxygen.

1969 7Up UnCola "Un & Un Is Too" 30" x 16" vintage billboard poster "Printer's Proof" by Kim Whitesides #7Upvintage
Dot & Cross Diagrams. Dot and cross diagrams are diagrams that show the arrangement of the outer-shell electrons in an ionic or covalent compound or element. The electrons are shown as dots and crosses. In a dot and cross diagram: Only the outer electrons are shown. The charge of the ion is spread evenly which is shown by using brackets.
Lewis electron dot structures are the diagrammatic representation of covalently bonded atoms in a molecule. They also show lone pairs of electrons that may exist in the molecule. They are used in the case of covalently bonded molecules as well as coordination compounds. This system was introduced by Gilbert Lewis in 1916.
CDOT Online Transportation Information System. This is the access point to information frequently used for transportation planning and project development. Information is provided on current and projected traffic volumes, state highway attributes, summary roadway statistics and geographic data.
Jan 15, 2022 · Dot Sequence Diagram Example Dot Sequence Diagram Template Graphviz Dot Sequence Diagram. Sequence Diagram. Once installation is complete you should first try creating some Sequence Diagrams. Since these work without Graphviz this is the fastest way to check that the installation worked. If they do not work, other diagrams will probably not ...
CDOT OUTDOOR ADVERTISING DIAGRAMS Figure 1A. Cotton Areas by Zoning 1 Figure 1B. Cotton Areas Coincident w/ Roadway R.O.W. 2 Figure 1C. Cotton Areas w/ Roadway R.O.W. Angled 3 Figure 1D. Cotton Areas w/ Offset Roadway R.O.W. 4 Kerr Area Exception 5 Sign Spacing Details CDOT OUTDOOR ADVERTISING DIAGRAMS Figure 1A.

1971 21'x10' 7Up UnCola "Like No Cola Can" vintage electric rainbow billboard poster by Milton Glaser (Push Pin Studios era) (now Mad Men Season 7 poster artist), Electric Rainbow #7Upvintage
Contact Staff Bridge Branch 2829 W. Howard Place Denver, CO 80204 303-757-9309. Lynn Croswell, P.E. Bridge & Structure Inspection Engineer
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons:
Transcribed image text: Chemical Formula: Central Atom: Co Dot Diagrams: Electrons: 10 valence elcctrons Lewis Structure: Central Atom: Chemical Formula: N H3 Electrons: Dot Diagrams: Lewis Structure:
Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen.

The Tao of the Sky in the Eye: Cosmic Background Radiation Anomalies and Asymmetries of the Visual Field
The notable exceptions are the compounds CN (cyanide) and CO (carbon monoxide), which the bond is polar due to the unequal sharing of electrons between the two atoms in the structure. The structures do not look like the lewis dot structure for CO2, where the carbon does not have any lone pair electrons on the carbon.
The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. Answer : Lewis-dot structure : It represent the number of valance electrons present in ...
Loop fragment is used to represent a repetitive sequence. Place the words ‘loop’ in the name box and the guard condition near the top left corner of the frame. Sequence Diagram: It is used to surround the whole sequence diagram. Example of a Sequence Diagram. An example of a high-level sequence diagram for online bookshop is given below.
CO2 or carbon dioxide is considered as acid or can even be called Lewis Acid. The resonance structure accepts lone pairs of electrons, but the three lone pairs of electrons are in the oxygen molecule. There are no lone pairs of electrons in CO 2. Although, when dissolved in water, CO 2 takes the form of carbonic acid using this formula.



![[NL_9849] Dot Diagram Of Co2 Free Diagram](https://static-assets.imageservice.cloud/6824400/dot-cross-diagram-of-co-wiring-diagram-imp.jpg)















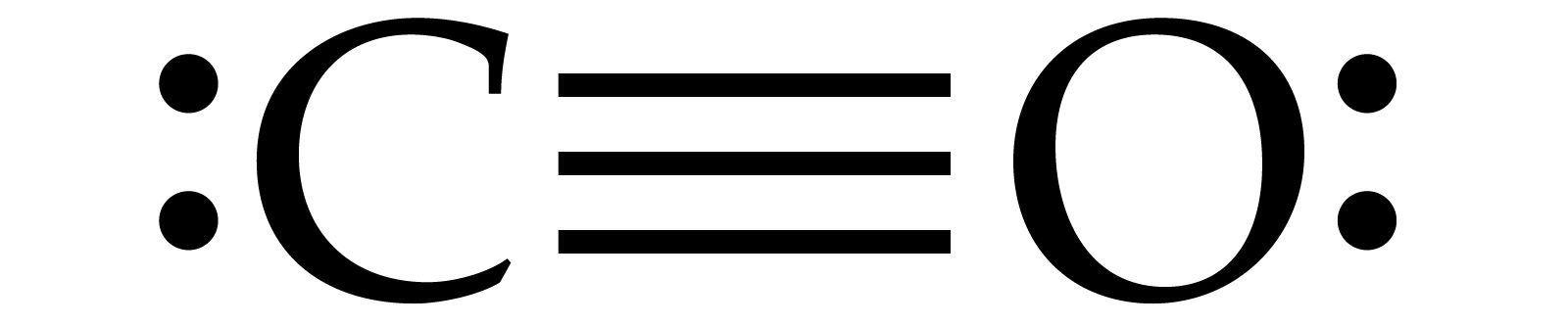


![Solved: In The Following Lewis Structure Of [CO3]2-, Every ...](https://media.cheggcdn.com/media%2F1ab%2F1ab7de8d-eca0-494e-9418-0bd1593960e0%2FphpM8jzx1.png)



![[NL_9849] Dot Diagram Of Co2 Free Diagram](https://static-assets.imageservice.cloud/6824413/draw-the-lewis-dot-structure-for-co2-how-to-draw-the-lewis-structure.jpg)





Comments
Post a Comment