42 h2 electron dot diagram
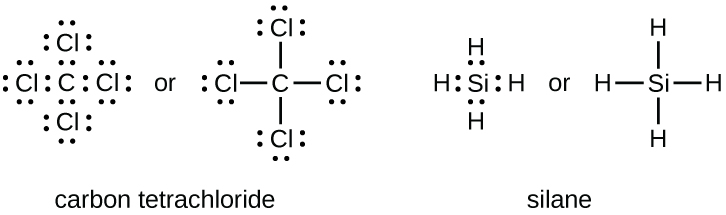
In a previous chapter, you learned that the valence electrons of an atom can be shown in a simple way with an electron dot diagram. A hydrogen atom is shown ... The H2 Se Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the H2 Se molecule.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
H2 electron dot diagram
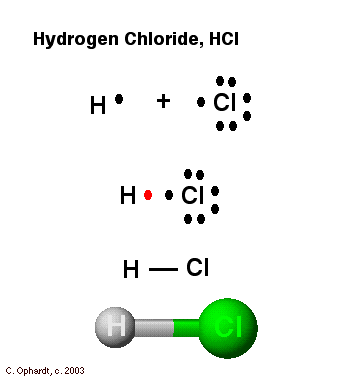
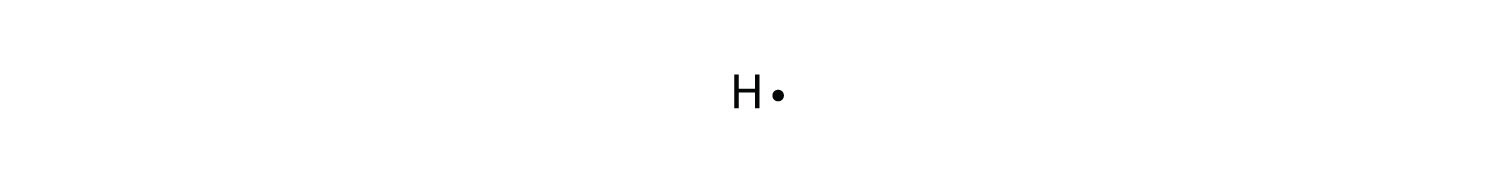
A step-by-step explanation of how to draw the H2 Lewis Dot Structure (Hydrogen gas).For the H2 structure use the periodic table to find the total number of v... What is the Lewis dot diagram for H2? Wiki User. ∙ 2011-10-22 00:12:10. See Answer. Best Answer. Copy. I'm pretty sure it's just H-H due to each hydrogen only having one valence electron. I ... H2o2 Dot Diagram H2o2 Dot Diagram The chemical name for H2 O2 is hydrogen peroxide. Its Lewis structure shows us where the valence electrons are located in the molecule, which can aid us in. Count the number of electrons, add single bonds between the atoms, using two electrons per bond, arrange the remaining electrons around the.
H2 electron dot diagram. To use dot diagrams to show how electrons can be transferred or shared to form bonds. ICl NH3 MgS H2O PCl3. MgCl2 CO2 NaCl Na2O CH4 CHCl3 O2 H2 HCl OH- Directions: Draw electron dot diagrams for each element in the compounds above. Draw only the valence electrons. Put one electron on each of the "4 sides" first, before pairing them up. wt i.h electron dot diagram represents H2? (1) H. H (3):H.H: @; H:n Look at the electron dot formula shown below. The attraction of X for the bondinq electrons,,^,,culd be greatest ,rvhen X ,"pr"t"-ntt .n atom of (1) s (3) se (a) Te Nletallic attractive forces - There is a crystalline lattice, where positive ions valence elec-tib-ns are free to ... (B) Fluorescence resonance energy transfer (FRET) spectra generated under different conditions. a: H1 + H2. b: miR-1246 + H1 + H2. c: piR-651+ H1 + H2. d: H0 651 + H1 + H2. e: H0 1246 + H1 + H2. f: miR-1246 + H0 1246 + H1 + H2. g: piR-651 + H0 651 + H1 + H2. The initial concentrations are the same as in (A) except for piR-651 and miR-1246 both at 1 nM, and H1 at 50 nM. (C) … Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.
Feb 22, 2019 - A step-by-step explanation of how to draw the H2 Lewis Dot Structure (Hydrogen gas).For the H2 structure use the periodic table to find the total number of v... Which Electron Dot Diagram Represents H2 The left diagram shows a Lewis dot structure of sodium with formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed. We can use Lewis dot formulas to show covalent bond formation. 1. H. 2 molecule. +. H. H. H H.. or H2. H. Cl . The Lewis Dot Structure for H 2:. Molecular hydrogen (H 2) is a flammable gas.The Lewis structure is quite simple to draw, and shows how each H atom contributes it's valence electron in making the ... Academia.edu is a platform for academics to share research papers.
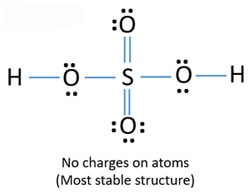
H2o2 Dot Diagram 06.10.2018 7 Comments Draw a Lewis dot diagram for H2O2 (hydrogen peroxide), and use the oxidation- state method of electron bookkeeping to determine how many electrons each. The chemical name for H2 O2 is hydrogen peroxide. Science Chemistry Q&A Library 3-bromo-1-pentene and 1-bromo-2-pentene undergo SN1 reaction at almost the same rate, but one is a secondary halide while the other is a primary halide. Explain your answer. Then, decide which the following compounds will react faster in an E2 reaction; trans-1-bromo-2-isopropylcyclohexane or cis-1-bromo-2-isopropylcyclohexane. Based on the best Lewis electron-dot structure for SO42- and formal charge considerations, what is the predicted S-O bond order for each S-O bond? 1.5 Shown below is a model of PF5 having an orientation in which one or more atoms are hidden from view. The difference between the Lewis dot structure and the structuralformula is that the formula only shows the bonds that have formedwhereas the dot structure shows all the valen ce electrons,including lone pairs, in that molecule. Drawing the Lewis Structure for C 2 H 2 (Ethyne or Acetylene) For C 2 H 2 you have a total of 10 valence electrons to ...
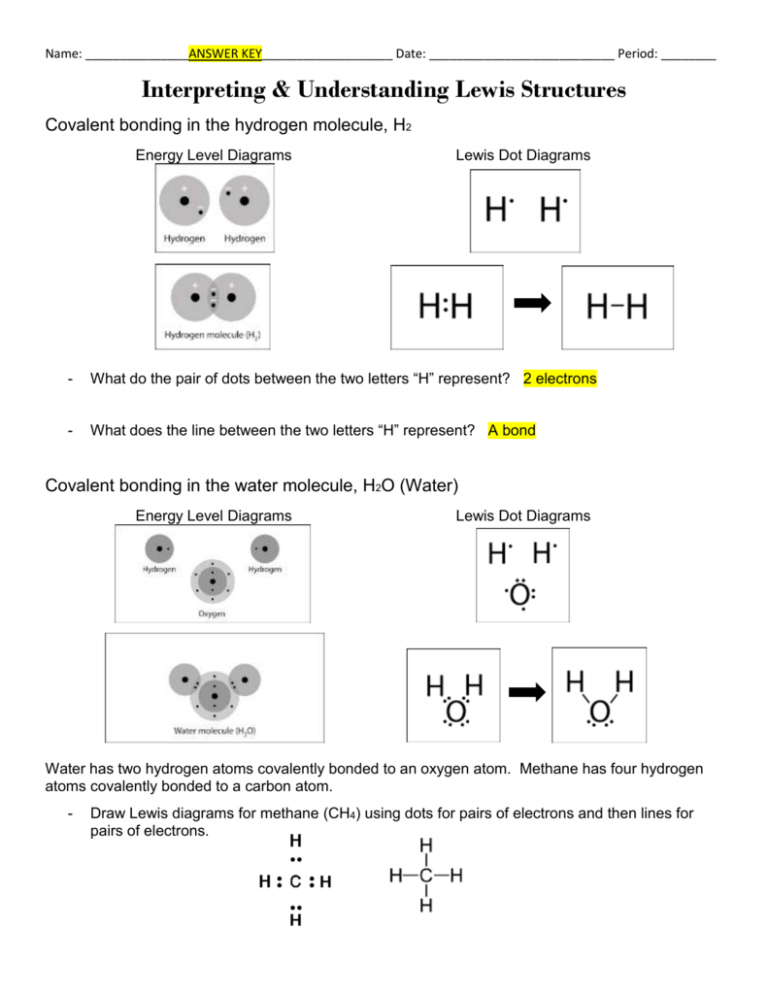
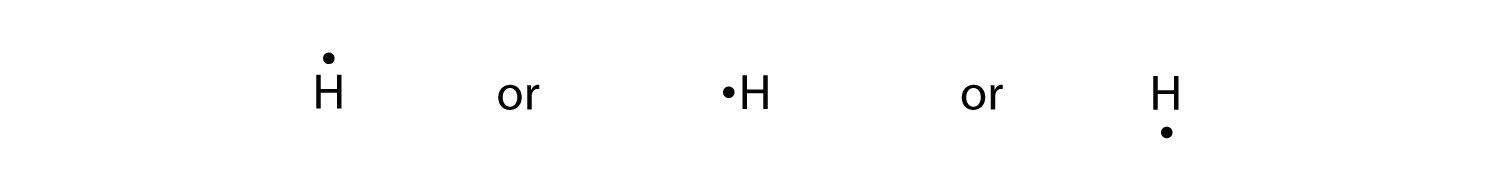
What is the Lewis dot structure of H2? A hydrogen atom is shown as H• because of its one valence electron. The structures of molecules that are held together by covalent bonds can be diagrammed by Lewis electron-dot structures. The hydrogen molecule is shown in Figure 1.1.
Lewis electron-dot diagrams for CO2 and SO2 are given above. The molecular geometry and polarity of the two substances are A. the same because the molecular formulas are similar B. the same because C and S have similar electronegativity values C. different because the lone pair of electrons on the S atom make it the negative end of a dipole
19 May 2021 — draw the electron dot diagram and structure of a hydrogen b chlorine c oxygen d nitrogen - Chemistry - TopperLearning.com | j0tirjrr.
Lewis Dot - 13 images - how is the lewis structure of sulfuric acid determined, lewis dot diagram and octet rule youtube, lewis dot structure of h3po4 phosphoric acid youtube, lewis dot basic youtube,
Draw The Electron Dot Structure Of H2. Draw the electron dot structure of H2. The electron dot structure or Lewis structure of H2 (H-H) is given below: Was this answer helpful? 4 (1) (2) (1) Choose An Option That Best Describes Your Problem. Answer not in Detail. Incomplete Answer. Answer Incorrect. Others.
25 Jun 2021 — A hydrogen atom is shown as H⋅ because of its one valence electron. The structures of molecules that are held together by covalent bonds can be ...
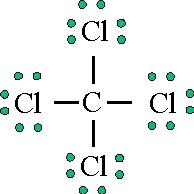
The Lewis dot structure for the Chlorine atom is as follows- 2. Choose a suitable central atom for the compound. The central atom is supposed to be the least electronegative one out of the constituent atoms. The central atom is supposed to share its electron density with all other atoms. Chlorine is a diatomic molecule.
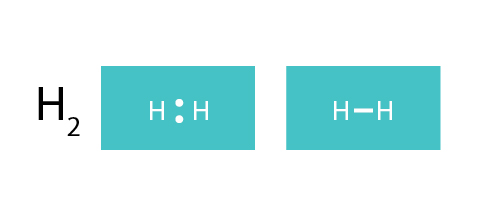
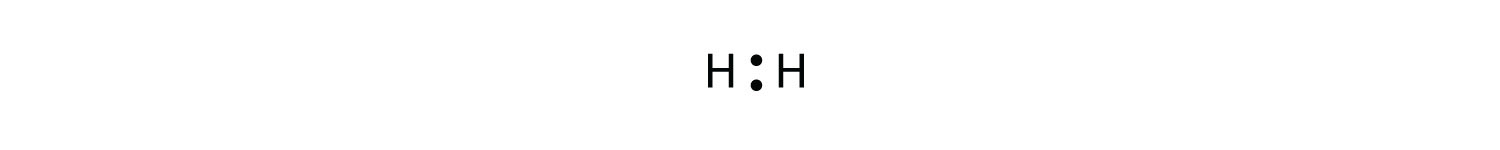
This is the electron dot diagram for the H2 molecule: H:H Using numbers, complete the following statement. In the hydrogen molecule, there are bonding electron pair(s) and lone electron pair(s). Check Answ. This is the electron dot diagram for the F2 molecule: Using numbers, complete the following statement In the fluorine molecule, there are bonding
Lewis electron dot-diagrams for CO2 and SO2 are given above. the molecular geometry and polarity of the two substances are. ... because pure H2 is a hazardous substance, safer and more cost effective techniques to store it as a solid for shipping purposes has been developed. One such method is the reaction represented above, which occurs at 200C.
The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol.
What is the dot structure of H2? On the left is a single hydrogen atom with one electron. On the right is an H2 molecule showing the electron cloud overlap. The shared pair of electrons is shown as two dots in between the two H symbols (H:H). This is called a single covalent bond, when two atoms are joined by the sharing of one pair of electrons.
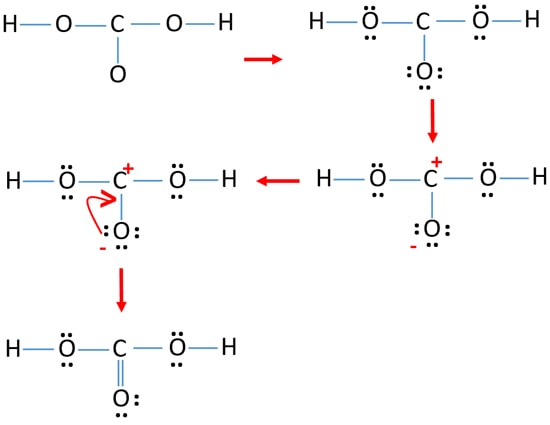
Vor 2 Tagen · As we see in the above-mentioned diagram, the five molecules have fulfilled their octet configuration and the total valence electron number remains 32. By drawing single bonds, we get the structure as: Step 5: Formal Charge Calculation. We may think from the above sketch that we have found our perfect Lewis Structure but there is one step left.
Click here👆to get an answer to your question ️ Draw the electron dot structure for oxygen molecule. Solve Study Textbooks. Join / Login >> Class 10 >> Chemistry >> Carbon and its compounds >> Covalent bonding in carbon compounds >> Draw the electron dot structure for oxyg. Question .
30.12.2021 · Redox reactions of aqueous colloidal TiO2 4 nm nanoparticles (NPs) have been examined, including both citrate-capped and uncapped NPs (c-TiO2 and uc-TiO2). Photoreduction gave stable blue colloidal c-TiO2R NPs with 10–60 electrons per particle. Equilibration of these reduced NPs with soluble redox reagents such as methylviologen …
Let's do the Lewis structure for H2, Hydrogen gas. It's a quite explosive gas, so please don't fill your blimp up with it. Let's look at the periodic table. Hydrogen is in group 1, that means it has 1 valence electron. But we have 2 Hydrogen atoms, so let's multiply that by 2, for a total of 2 valence electrons.
h2 lewis structure how to draw the dot structure for h2. Hydrogen Lewis Structure. Here are a number of highest rated Hydrogen Lewis Structure pictures upon internet. We identified it from reliable source. Its submitted by management in the best field. We put up with this kind of Hydrogen Lewis Structure graphic could possibly be the most ...
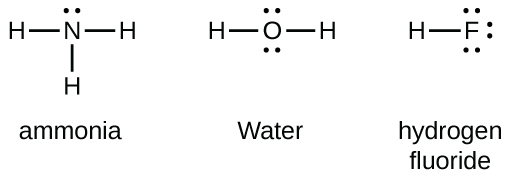
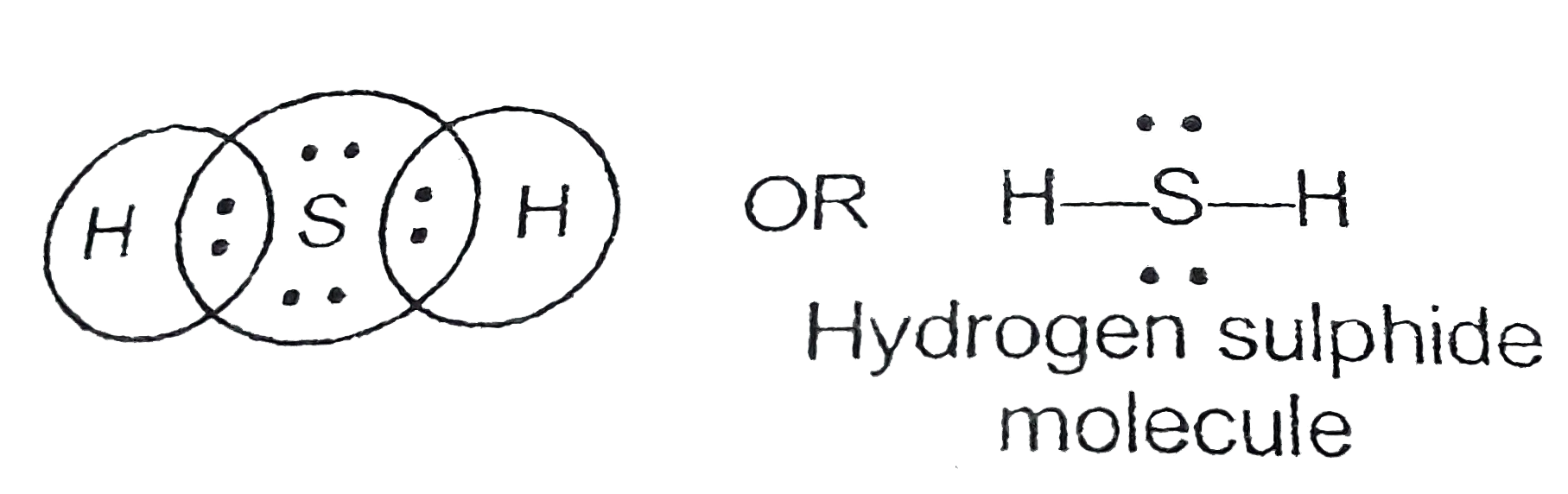
Draw the electron dot structures for (a) ethanoic acid (b) H 2 S (c) propanone (d) F 2 Answer: Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known. it defines the nature of bond and position of atoms of the molecule which are connected in the molecule.
dot electron diagram h2 formula which structural lewis structure hydrogen draw covalent molecule chemical represents water hydroxide ionic oh . lewis dot structure electron diagram h2o structures co2 covalent carbon polar bonds following bond electrons pairs bonding valence dioxide struttura .
Draw Lewis dot diagram for the following. Hydrogen (H2) Maharashtra State Board HSC Science (General) 11th. Textbook Solutions 8028. Important Solutions 18. Question Bank ... Draw Lewis dot diagram for the following. Hydrogen (H 2) Advertisement Remove all ads. Solution Show Solution. or.
Check the Formal Charges to make sure you have the best Lewis Structure. Explain How Examples: SO 4 2-, N 2 O, XeO 3; Notable Exceptions to the Octet Rule. H only needs 2 valence electrons. Be and B don't need 8 valence electrons. S and P sometimes have more than 8 val. Electrons.
A step-by-step explanation of how to draw the H2 Lewis Dot Structure (Diatomic Hydrogen).Note that Diatomic Hydrogen is often called Molecular Hydrogen or ju...
Due to the unique molecular structure, H2 can easily target the mitochondria, suggesting that H2 is a potential antagonist of mtROS-dependent NLRP3 inflammasome activation. Here we have showed that, in mouse macrophages, H2 exhibited substantial inhibitory activity against LPS-initiated NLRP3 inflammasome activation by scavenging mtROS.
C2H2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw lewis structure of C2H2.
A semiconducting quantum dot (like CdSe, CdTe etc) is a portion of semiconductor whose exciton (electron-hole quasiparticle) are Confined in all three spatial dimensions. Properties of these ...
16.12.2021 · Please send to johnspray274
Sep 24, · Lewis dot structures for a nonpolar molecule must show equal electron distribution throughout the molecule, thus having no dipole. For a simple example: H2 is nonpolar. So H-H would be represented with H (two dots)H. 2. Fluorine has 7/8 valence electrons, therefore F is surrounded by 3 sets of pairs of dots and 1 single dot on one ...
H2o2 Dot Diagram H2o2 Dot Diagram The chemical name for H2 O2 is hydrogen peroxide. Its Lewis structure shows us where the valence electrons are located in the molecule, which can aid us in. Count the number of electrons, add single bonds between the atoms, using two electrons per bond, arrange the remaining electrons around the.
What is the Lewis dot diagram for H2? Wiki User. ∙ 2011-10-22 00:12:10. See Answer. Best Answer. Copy. I'm pretty sure it's just H-H due to each hydrogen only having one valence electron. I ...
A step-by-step explanation of how to draw the H2 Lewis Dot Structure (Hydrogen gas).For the H2 structure use the periodic table to find the total number of v...









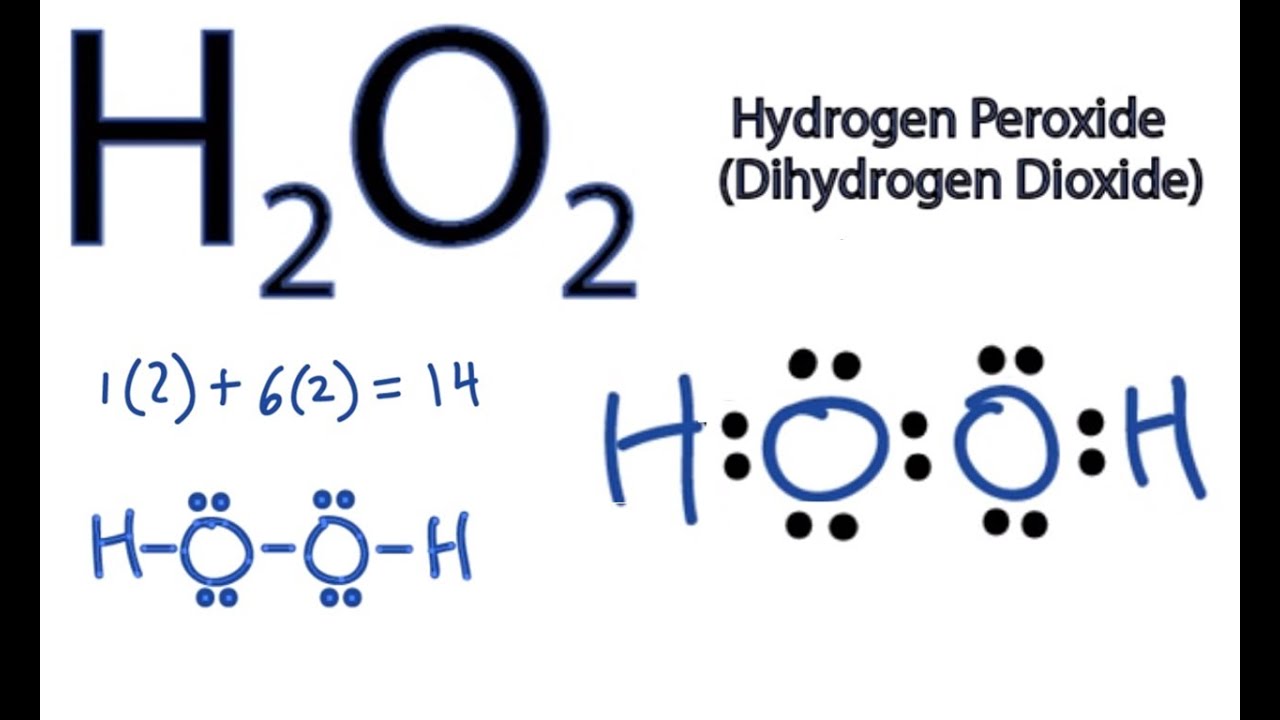




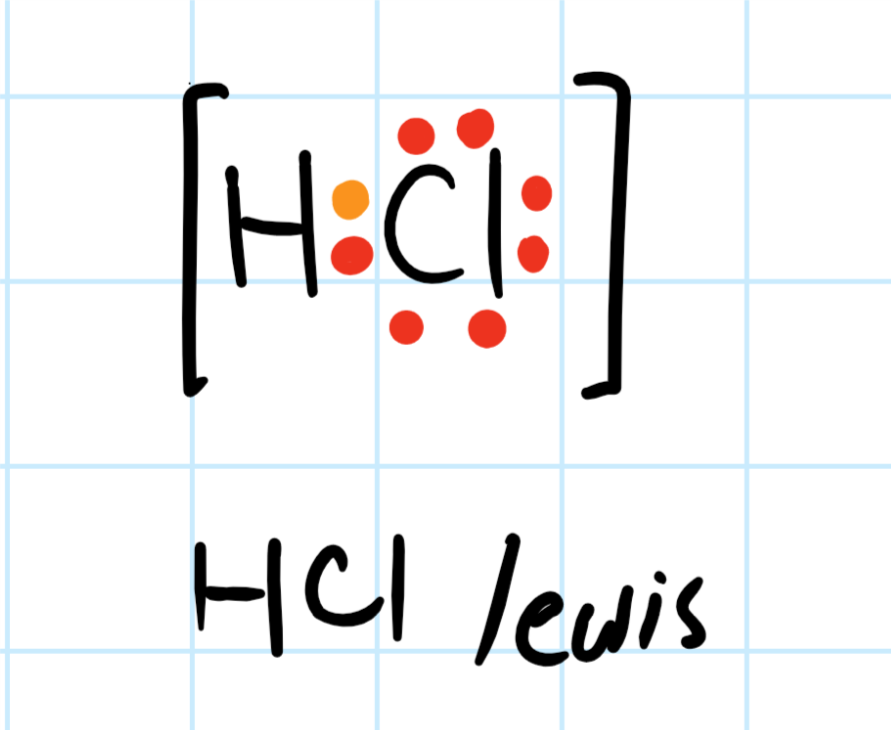

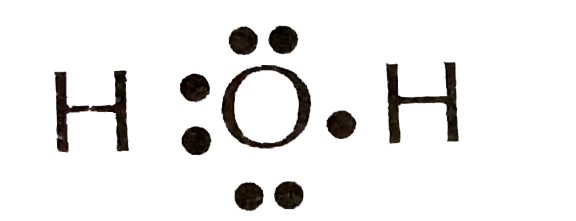
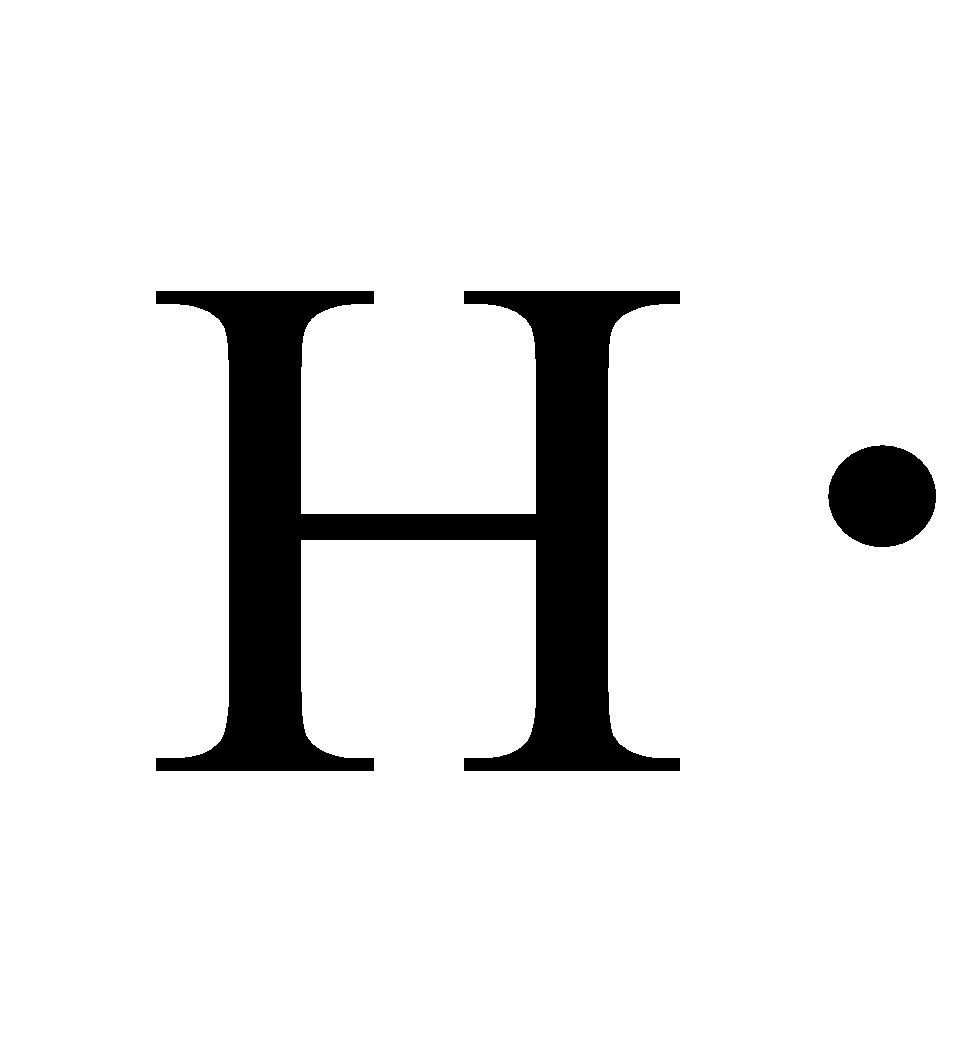






Comments
Post a Comment