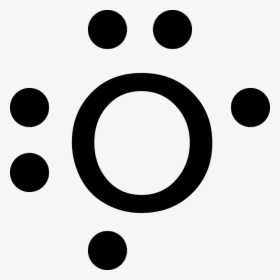
43 dot diagram for oxygen
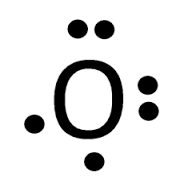
Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds. The valence electrons are written as dots surrounding the symbol for the element: one dot is place on each side first, and when all four positions are filled, the remaining dots are paired ... Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
Shortcuts & Tips · Problem solving tips · Important Diagrams · Memorization tricks · Common Misconceptions · Mindmap · Cheatsheets.1 answer · Top answer: Option B shows the lewis structure of O2 molecule.

Dot diagram for oxygen
The lewis dot structure of the oxygen atom can be represented by the pictorial diagram by showing the number of valence electrons with dots around the symbol of the oxygen. Answer and Explanation:... For diatomic oxygen, the Lewis dot structure predicts a double bond. While the Lewis diagram correctly predict that there is a double bond between O atoms, it incorrectly predicts that all the valence electrons are paired ( i.e., it predicts that each valence electron is in an orbital with another electron of opposite spin). O_2 would have a double bond. Both atoms of oxygen in the O_2 molecule have 6 valence electrons. Each oxygen atom looks like this: They each have two lone electrons which need to be paired up. So, the two lone electrons from one oxygen atom bond with the two lone electrons from the other oxygen atom, forming a double bond. The Electron Dot Diagram for O_2 looks like this:
Dot diagram for oxygen. Draw the Lewis Dot Structure for Oxygen. Since Oxygen is in Period 2, it can fit a maximum of eight (8) electrons second energy level. Oxygen Group VI, which means it has a total of six (6) valence electrons around the atom Example A. Determine the total number of valence electrons for C Carbon is in Group IV, 4 valence electrons Oxygen needs to bond twice, shown as the lone dots on the left and right sides of the oxygen atoms in the below diagram. There are also two pairs of dots, ... In the Lewis-dot structure the valance electrons are shown by 'dot'. The given molecule is, As we know that rubidium has '1' valence electrons, iodine has '7' valence electrons and oxygen has '6' valence electrons. Therefore, the total number of valence electrons in = 1 + 7 + 2 (6) = 20 The electron dot diagram for an element shows the valence electrons for the element. This is the Lewis Dot Structure for O2 commonly referred to as oxygen gas. Four of the valence electrons exist in lone pairs implying that the oxygen atom must participate in two single bonds or one double bond in order to attain an octet configuration.
May 30, 2018 · The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol. There are also two more valence electrons and they are paired with two of the unpaired electrons. Electron dot diagram of the Oxygen atom Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Oxygen, we got to know, it has 6 valence electrons. So, just represent these 6 valence electrons around the Oxygen atom as a dot. The Electron configuration of Oxygen Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. A step-by-step explanation of how to draw the O2- Lewis Dot Structure.For the O 2- structure use the periodic table to find the total number of valence elect...
Oxygen atoms need to gain 2 electrons each. (2.6) The common multiple of 2 and 3 is 6. It takes two Aluminium atoms to lose 6 electrons. And three Oxygen atoms to gain them. The first diagram is okay, although an examiner might want to see dots and crosses rather than just dots. Nov 30, 2018 · Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Transcript: This is the O2 2- Lewis structure. For the peroxide ion, Oxygen has six valence electrons. Oxygen difluoride appears as a colorless poisonous gas with a strong peculiar odor. Highly toxic by inhalation. Corrosive to skin and eyes. Can explode on contact with water.Decomposes to toxic gaseous fluorine if heated to high temperature. Prolonged exposure of the containers to high heat may result in their violent rupturing and rocketing. Click here👆to get an answer to your question ️ Draw the electron dot structure for oxygen molecule. Solve Study Textbooks. Join / Login >> Class 10 >> Chemistry >> Carbon and its compounds >> Covalent bonding in carbon compounds >> Draw the electron dot structure for oxyg. Question .
Photo: Benjah-bmm27 via Wikimedia Commons, Public Domain. O2 is an allotrope of oxygen and is made out of two oxygen atoms bound together. Although the chemical formula for this allotrope is O2, it is frequently just referred to as oxygen.O2 or dioxygen’s particular formulation is one of the most common elemental compounds on the planet, constituting around 20.8% of the Earth’s atmosphere.
We can use dot and cross diagrams to show how a pair of electrons forms a covalent bond. Here is the dot and cross diagram for oxygen (O2), a diatomic molecule. Notice the lone pairs of electrons...
Two atoms of oxygen (2.6) combine to form the molecules of the element oxygen O 2 (only the outer shell of oxygen's electrons are shown).. Each oxygen atom is two electrons short of a full outer shell, so each oxygen atom shares two of its electrons with the other atom, so both oxygen atoms have a full outer shell. (Lewis diagram of oxygen) simplified 'dot and cross' electronic diagram for the ...
What is the Lewis dot structure for oxygen ion? Each O is surrounded by four dots and two sticks or lines, representing another 4 electrons in the O2 double bond. So each O is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter O's in the O2 Lewis structure represent the nuclei (centers) of the ...
Dot- and - cross diagram of oxygen atom Question: Draw a dot- and- cross diagram for calcium atom, showing the electrons in the outermost shells. Calcium is in Group II --> 2 valence electrons.
Lewis Structure (electron dot diagram) for the oxygen molecule, O 2, OR . There are 2 bonding pairs of electrons shared between the 2 oxygen atoms, and each oxygen atom also has 2 lone pairs (non-bonding) pairs of electrons. In the Valence structure for the oxygen molecule, each bonding pair of electrons is replaced by a dash (-) to represent a ...
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
The dot and cross diagram shows that a water molecule is made up of one oxygen atom joined to two hydrogen atoms, so the formula of water is H 2 O. Double and triple bonds
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen)).For the O2 structure use the periodic table to find the t...
In order to determine the Lewis dot structure of the ${{O}_{2}}$, we use the octet rule, that is, there should be 8 valence electrons in the outermost shell of an atom. An element follows the octet rule to obtain a stable state. In case of oxygen atoms present in group 16 of the periodic table.
(d) Xenon can react with oxygen and fluorine to form compounds such as XeO 3 and XeF 4. In the boxes provided, draw the complete Lewis electron-dot diagram for each of the molecules represented below. XeO 3 XeF 4 One point is earned for each correct Lewis electron-dot diagram. Omission of lone pairs of electrons on the O or F atoms
Lewis Electron Dot Structure for the molecule: HCOOH (Formic Acid) In the first step use four electron pairs to form single bonds between neighbouring atoms. Except for hydrogen, complete the octet of each atom by adding two lone pairs on each oxygen atom and one oxygen atom gets double-bonded between C and O.
O_2 would have a double bond. Both atoms of oxygen in the O_2 molecule have 6 valence electrons. Each oxygen atom looks like this: They each have two lone electrons which need to be paired up. So, the two lone electrons from one oxygen atom bond with the two lone electrons from the other oxygen atom, forming a double bond. The Electron Dot Diagram for O_2 looks like this:
For diatomic oxygen, the Lewis dot structure predicts a double bond. While the Lewis diagram correctly predict that there is a double bond between O atoms, it incorrectly predicts that all the valence electrons are paired ( i.e., it predicts that each valence electron is in an orbital with another electron of opposite spin).
The lewis dot structure of the oxygen atom can be represented by the pictorial diagram by showing the number of valence electrons with dots around the symbol of the oxygen. Answer and Explanation:...


















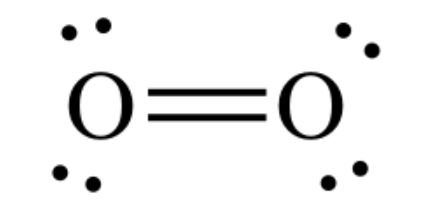




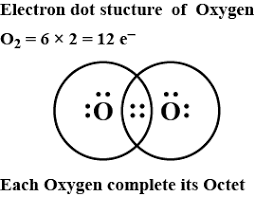

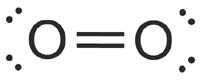





Comments
Post a Comment