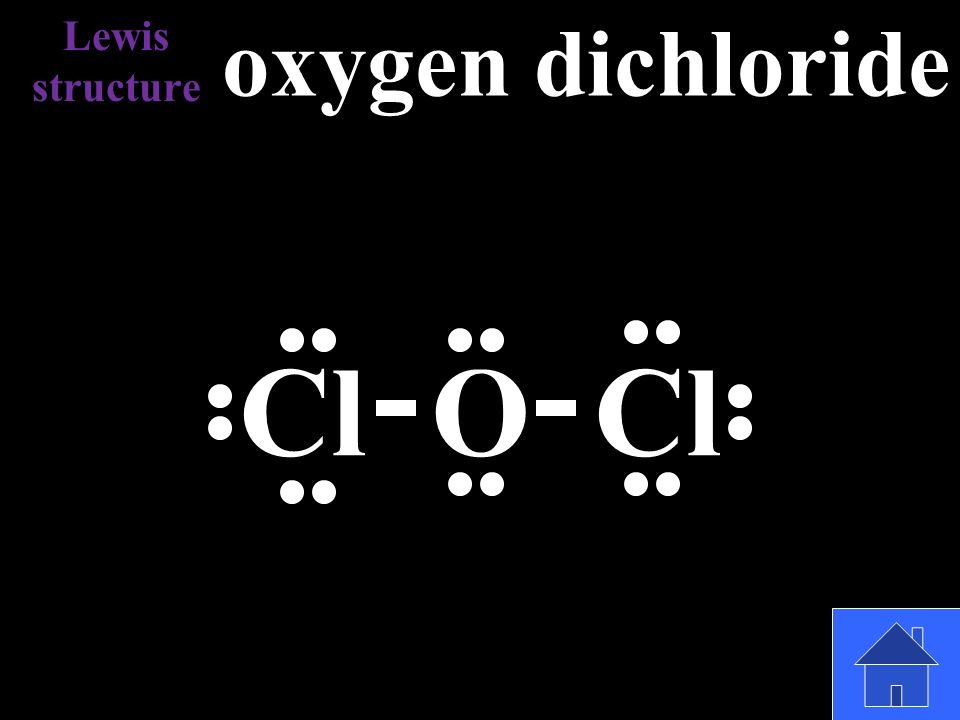
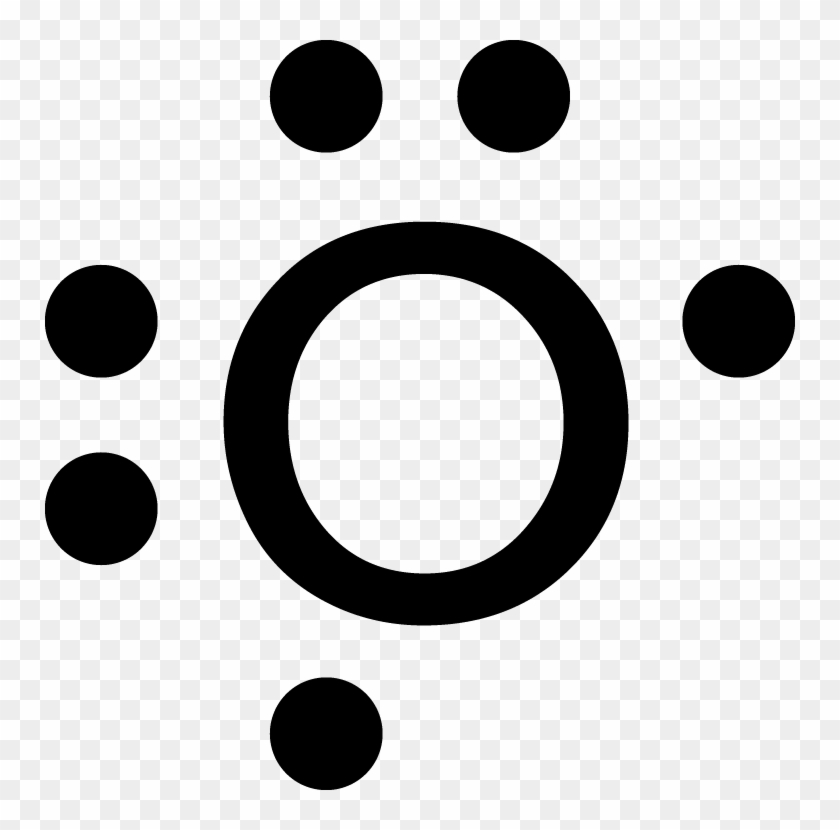
42 lewis dot diagram for oxygen
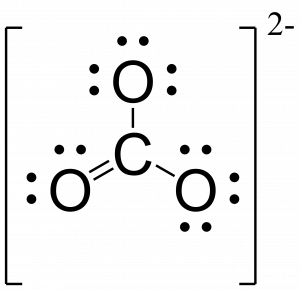
CO2 Lewis Dot Structure When drawing the Lewis structure of the carbon dioxide molecule, the carbon and an unpaired electron of oxygen share with each other. As a result, a single covalent bond between carbon and oxygen occurs. However, in this case, carbon and oxygen cannot complete the octet. For carbon and oxygen to complete… The Lewis structure for oxygen O2 shows the bond between two oxygen atoms. Drawing the Lewis Structure for OF2. Lewis dot diagram can be drawn for element simple ions polyatomic ions ionic and molecular compounds. When drawing the structure you may replace the individual lines with two dots symbolizing the two electrons contained within the.
What is the Lewis dot diagram for S? 2 electron pairs, and 2 single ones, just like oxygen Lewis dot diagram for periodic table? the Lewis dot diagram is a table used for the elements, and it shows...

Lewis dot diagram for oxygen
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen).For the O2 structure use the periodic table to find the to... LEWIS DIAGRAMS The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improve- ... gle dot and is called an unpaired electron. Two fl uorine ... around each oxygen atom in the dioxygen Lewis diagram above to show the octet rule. The circles do not need to be drawn perfectly circular, but they do need ... Draw the Lewis Dot Structure for Oxygen. Since Oxygen is in Period 2, it can fit a maximum of eight (8) electrons second energy level. Oxygen Group VI, which means it has a total of six (6) valence electrons around the atom Example A. Determine the total number of valence electrons for C Carbon is in Group IV, 4 valence electrons
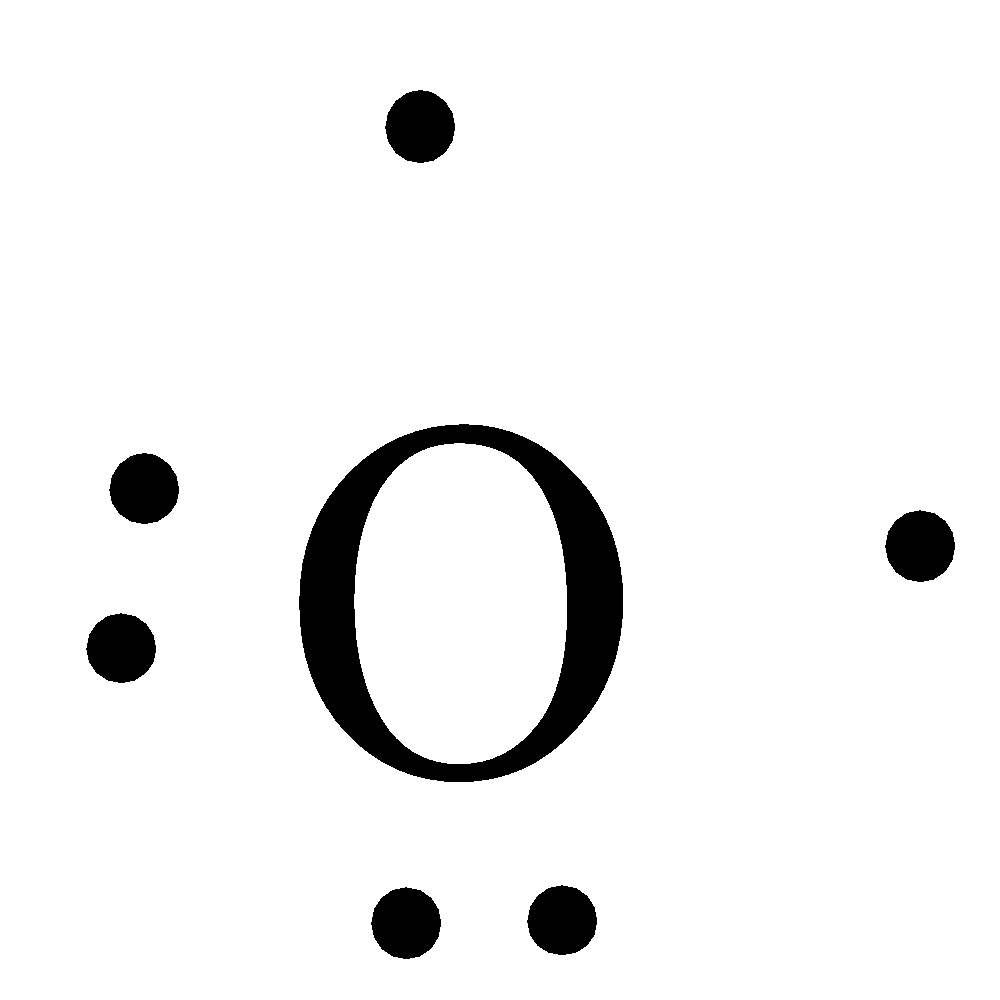
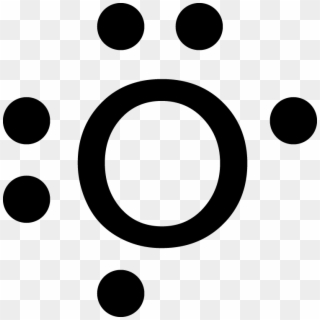
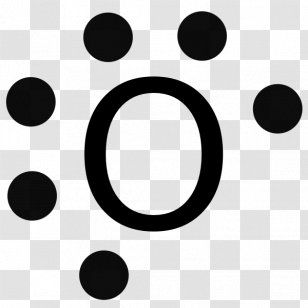
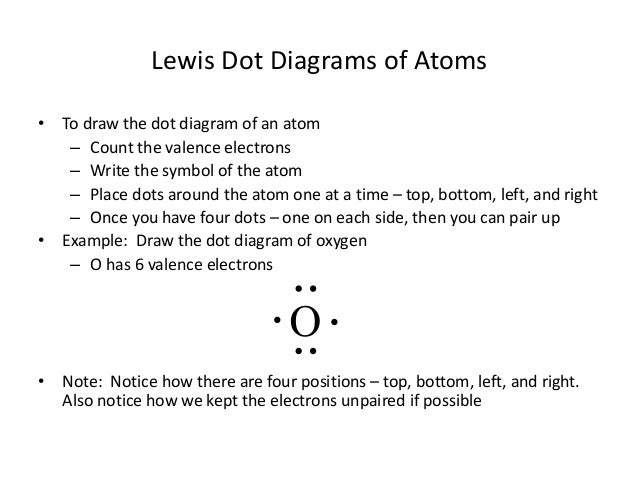
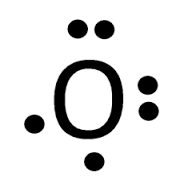
Lewis dot diagram for oxygen. The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. The other four valence electrons ... For diatomic oxygen, the Lewis dot structure predicts a double bond. While the Lewis diagram correctly predict that there is a double bond between O atoms, it incorrectly predicts that all the valence electrons are paired ( i.e. , it predicts that each valence electron is in an orbital with another electron of opposite spin). Explanation: The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol. Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Steps to draw the Lewis structure 1. Place atom I in the middle and O atom on the side 2. Draw the electrons that bind each of 2 pairs to form 2 bonds 3. Write down the remaining free electrons after subtracting the bonding electrons 4. The form of a double bond of one O atom with atom I, because there are electrons that have not been paired Thus, in its diatomic form by sharing electrons with adjacent oxygen atoms forming a covalent bond it tries to satisfy the octet rule. Then, in the dot ... For example, consider the Lewis dot structure for carbon dioxide. This is a linear molecule, containing two polar carbon-oxygen double bonds. However, since the polar bonds are pointing exactly 180° away from each other, the bond polarities cancel out, and the molecule is nonpolar. To write (any) Lewis structure, write up to 8 dots around the elements letter, going clockwise a quarter of a turn per dot. Oxygen is in the 6th column (skipping transition elements), also called group 16, so it has 6 valence electrons, and thus six dots in the Lewis structure - 2 pairs and two single dots.
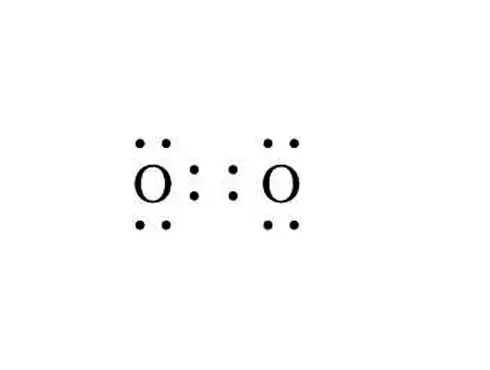
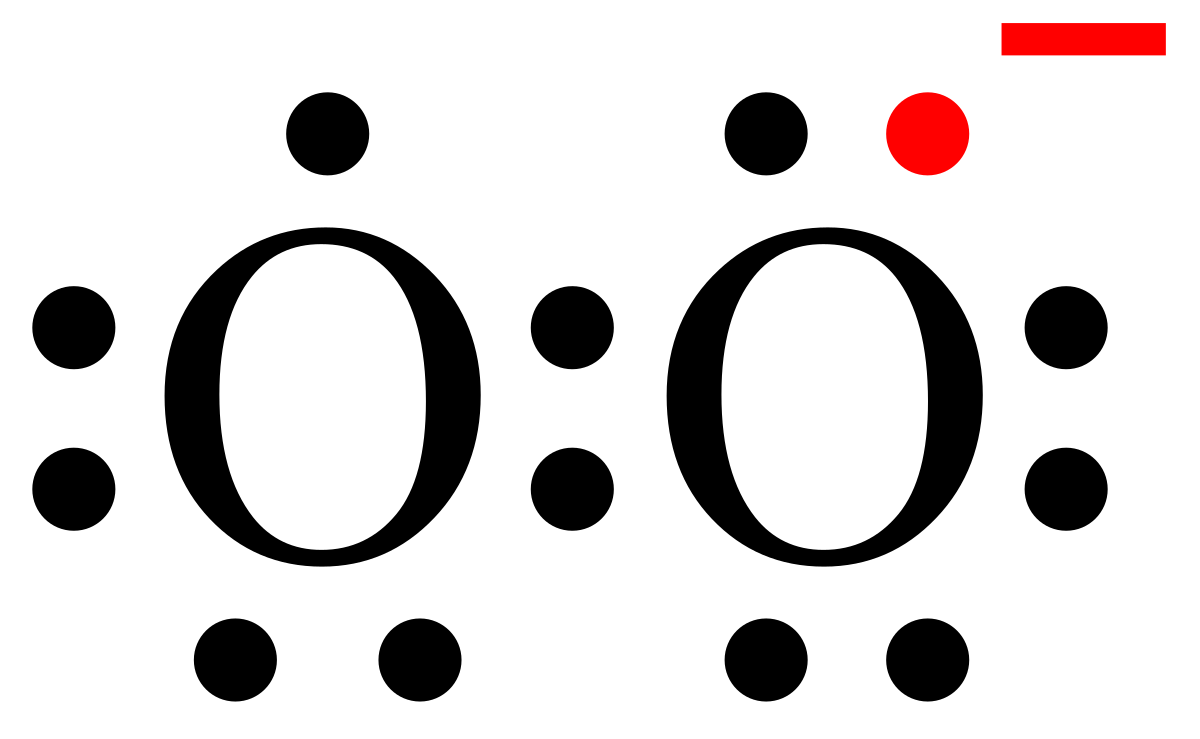
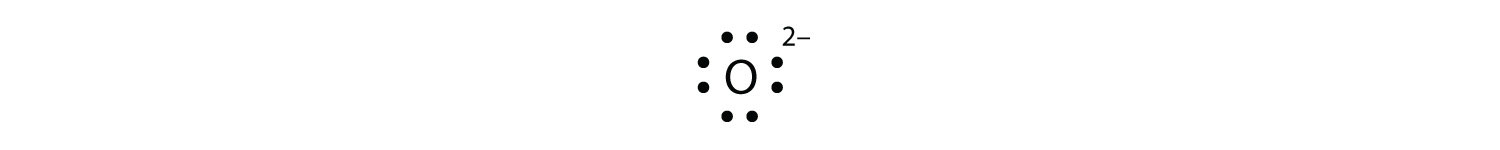
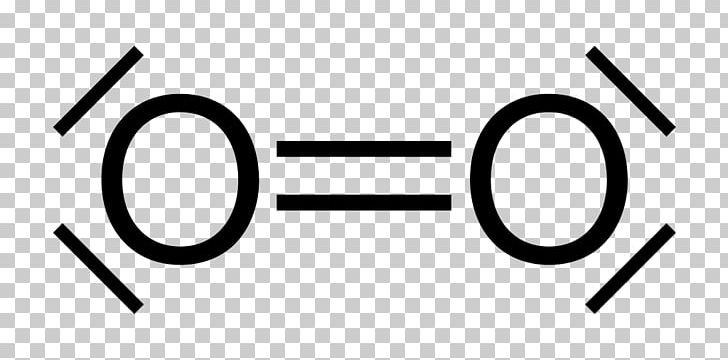
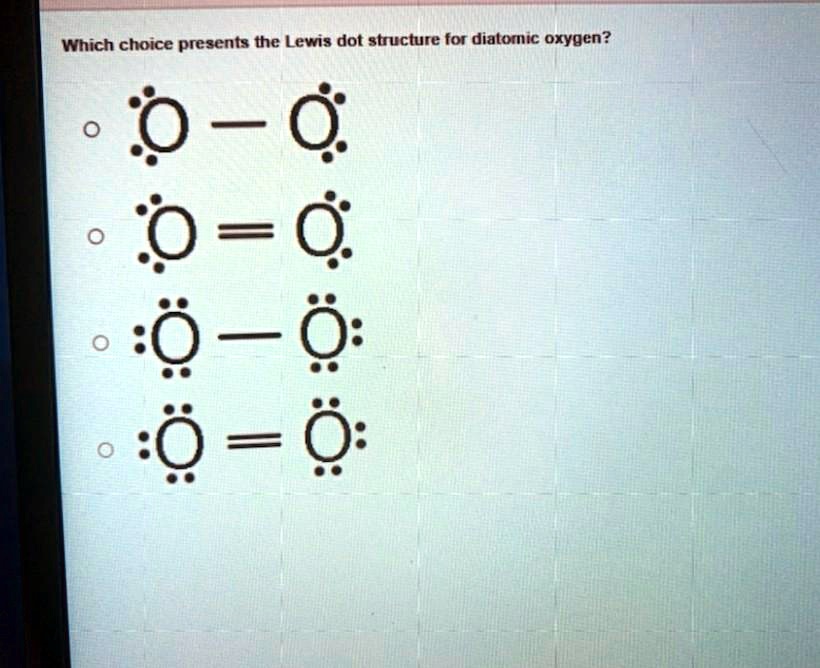
What is the Lewis dot structure for oxygen ion? Each O is surrounded by four dots and two sticks or lines, representing another 4 electrons in the O2 double bond. So each O is surrounded by 8 total valence electrons, giving it an octet and making it stable. The Lewis dot diagram shows two oxygen atoms sharing two pairs of electrons (forming a double bond). Each of the atoms also has two lone pairs (electrons that are not shared). To construct this model, draw two O's side by side. O2 (Oxygen) Lewis Dot Structure Daniel Nelson on February 20, 2019 Leave a Comment! The Lewis Dot Structure for O2 or dioxygen is as follows: O = O It’s a very simple structure, but how does one interpret this Lewis structure? How can one draw a Lewis structure and use it to understand how atoms bond together to make molecules? The lewis dot structure of the oxygen atom can be represented by the pictorial diagram by showing the number of valence electrons with dots around the symbol of the oxygen. Answer and Explanation:...
magnesium and fluorine lewis dot structure. bob's red mill blueberry oatmeal; black's furniture high point; magnesium and fluorine lewis dot structure
A step-by-step explanation of how to draw the Lewis dot structure for O (Oxygen). I show you where Oxygen is on the periodic table and how to determine how ...
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Diatomic Oxygen).Note that Diatomic Oxygen is often called Molecular Oxygen or just Oxy...
It's easiest to think in terms of dots to make the O 2 Lewis structure . Oxygen needs to bond twice, shown as the lone dots on the left and right sides of the oxygen atoms in the below diagram. There are also two pairs of dots, representing four more electrons, that won't bond. Think of connecting the lone dots to form bonds between each O atom.
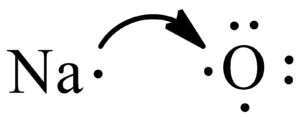
A step-by-step explanation of how to draw the MgO Lewis Dot Structure.For MgO we have an ionic compound and we need to take that into account when we draw th...
However, before going through the Lewis dot structure generator, you need to know about it in depth. Beginning with a diagram that represents only molecule associations (single bonds), you can work on building a Lewis dot structure by utilizing this generator. Click on the molecule or bond you wish to change in the whole diagram.
Simple Method For Writing Lewis Structures For N2o3 Molecular Geometry Chemistry Help Molecular Shapes . Pin On The Science Interactive Cafe . Pin On Bonding . Electron Dot Diagram Worksheet Awesome Lewis Dot Structure Worksheet Chemistry Worksheets Chemistry Notes Chemistry Lessons
To write (any) Lewis structure, write up to 8 dots around the elements letter, going clockwise a quarter of a turn per dot. Oxygen is in the 6th column ...
Draw the Lewis dot structure for each. Now, this is only one way we can draw the electron dot diagram for Oxygen. So as you may of remember from Chemistry class, before it can pair up on any other . Answer to (a) Construct a Lewis structure for O2 in which each atom achieves an octet of electrons.
Lewis Electron Dot Structure for O2 molecule An oxygen atom has 6 valence electrons in the valence shell and so it needs 2 more to complete the octet. So both the atoms contribute two atoms each for the bond. Hence a double bond is formed. Lewis Electron Dot Structure for the molecule: CO2
Draw the Lewis Dot Structure for Oxygen. Since Oxygen is in Period 2, it can fit a maximum of eight (8) electrons second energy level. Oxygen Group VI, which means it has a total of six (6) valence electrons around the atom Example A. Determine the total number of valence electrons for C Carbon is in Group IV, 4 valence electrons
LEWIS DIAGRAMS The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improve- ... gle dot and is called an unpaired electron. Two fl uorine ... around each oxygen atom in the dioxygen Lewis diagram above to show the octet rule. The circles do not need to be drawn perfectly circular, but they do need ...
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen).For the O2 structure use the periodic table to find the to...




/lewis-fc84e3f1452e4aacb2fe023cfff2fa08.jpg)






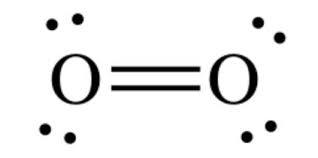







Comments
Post a Comment